Paclitaxel total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel (Taxol).[1] This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew (Taxus brevifolia). Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.
The paclitaxel molecule consists of a tetracyclic core called baccatin III and an amide tail. The core rings are conveniently called (from left to right) ring A (a cyclohexene), ring B (a cyclooctane), ring C (a cyclohexane) and ring D (an oxetane).
The paclitaxel drug development process took over 40 years. The anti-tumor activity of a bark extract of the Pacific yew tree was discovered in 1963 as a follow up of a US government plant screening program already in existence 20 years before that. The active substance responsible for the anti-tumor activity was discovered in 1969 and structure elucidation was completed in 1971. Robert A. Holton of Florida State University succeeded in the total synthesis of paclitaxel in 1994, a project that he had started in 1982. In 1989 Holton had also developed a semisynthetic route to paclitaxel starting from 10-deacetylbaccatin III. This compound is a biosynthetic precursor and is found in larger quantities than paclitaxel itself in Taxus baccata (the European Yew). In 1990 Bristol-Myers Squibb bought a licence to the patent for this process which in the years to follow earned Florida State University and Holton (with a 40% take) over 200 million US dollars.
Total synthesis
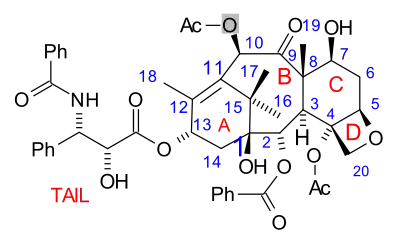
The total synthesis of taxol is called one of the most hotly contested of the 1990s [2] with around 30 competing research groups by 1992. The number of research groups actually having reported a total synthesis currently stands at 10 with the Holton group (article first accepted for publication) and the Nicolaou group (article first published) first and second in what is called a photo finish.
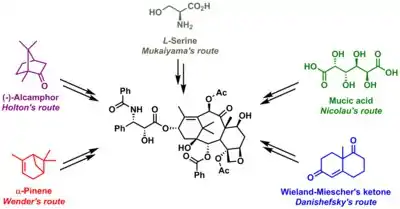
Some of the efforts are truly synthetic but in others a precursor molecule found in nature is included. The key data are collected below. What all strategies have in common is synthesis of the baccatin molecule followed by last stage addition of the tail, a process (except for one) based on the Ojima lactam.
- Holton Taxol total synthesis - year: 1994 - precursor: Patchoulol - strategy: linear synthesis AB then C then D - references: see related article [3][4]
- Nicolaou Taxol total synthesis - year: 1994 - precursor: Mucic acid strategy: convergent synthesis A and C merge to ABC then D - references: see related article[5]
- Danishefsky Taxol total synthesis - year: 1996 - precursor: Wieland-Miescher ketone strategy: convergent synthesis C merges with D then with A merges to ABCD - references: See related article
- Wender Taxol total synthesis - year: 1997 - precursor: Pinene strategy: linear synthesis AB then C then D - references:[6][7]
- Kuwajima Taxol total synthesis I. Kuwajima, - year: 1998 - precursor: synthetic building blocks strategy: linear synthesis A then B then C then D [8][9]
- Mukaiyama Taxol total synthesis - year: 1998 [10] - Precursor: L-serine strategy: linear synthesis B, then C, then A then D. References: see related article.
- Takahashi Taxol total synthesis - year: 2006 [11] - Precursor: geraniol strategy: convergent synthesis A and C merge to ABC then D
- Sato-Chida Taxol total synthesis - year: 2015, formal synthesis to a Takahashi intermediate [12][13][14]
- Nakada Taxol total synthesis - year: 2015, formal synthesis to a Takahashi intermediate [15]
- Baran Taxol total synthesis - year: 2020, total synthesis via a two-phase divergent synthetic approach.[16]
Ongoing research efforts are directed at the synthesis of taxadiene and taxadienone intermediates. The synthesis of related taxanes decinnamoyltaxinine E and taxabaccatin III has been reported [17]
Semisynthesis
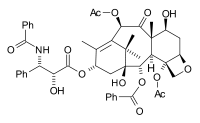
The commercial semisynthesis (by Bristol-Myers Squibb) of paclitaxel starting from 10-deacetylbaccatin III (isolated from the European yew) is based on tail addition of the so-called Ojima lactam to its free hydroxyl group:
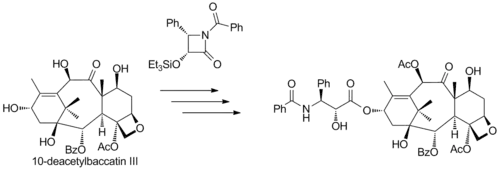
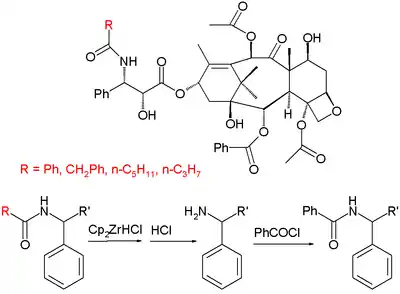
Another commercial semisynthesis (by the company Natural Pharmaceuticals) relies on the isolation of a group of paclitaxel derivatives isolated from primary ornamental taxanes. These derivatives have the same skeleton as paclitaxel except for the organic residue R of the terminal tail amide group which can be phenyl, or propyl or pentyl (among others) whereas in paclitaxel it is an explicit phenyl group. The semisynthesis consists of conversion of the amide group to an amine with Schwartz's reagent through an imine followed by acidic workup and a benzoylation.
In the production process Michigan grown yews which mature in 8 years are periodically topped and dried. This material is shipped to Mexico for a first extraction step (10% paclitaxel content) and then to Canada for further purification to 95% purity. The semisynthesis to final product takes place in China.[18]
Biosynthesis
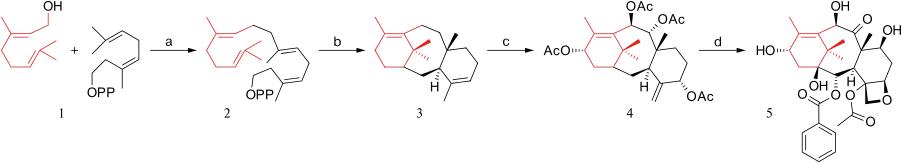
The biosynthetic pathway to paclitaxel has been investigated and consists of approximately 20 enzymatic steps. The complete scheme is still unavailable. The segments that are known are very different from the synthetic pathways tried thus far (Scheme 1). The starting compound is geranylgeranyl diphosphate 2 [19] which is a dimer of geraniol 1. This compound already contains all the required 20 carbon atoms for the paclitaxel skeleton. More ring closing through intermediate 3 (taxadiene) leads to taxusin 4. The two main reasons why this type of synthesis is not feasible in the laboratory is that nature does a much better job controlling stereochemistry and a much better job activating a hydrocarbon skeleton with oxygen substituents for which cytochrome P450 is responsible in some of the oxygenations. Intermediate 5 is called 10-deacetylbaccatin III.
A biochemical kilogram-scale production of taxadiene was reported using genetically engineered E. coli in 2011.[20]
References and notes
- Note that the original publications about the total synthesis use the name "taxol", which used to be the generic name before it was accepted as a trademark in 1992.
- Nina Hall (2003) "Creating complexity – the beauty and logic of synthesis" Chem. Commun. 2003 (6), 661-664. doi:10.1039/b212248k
- Robert A. Holton; Carmen Somoza; Hyeong Baik Kim; Feng Liang; Ronald J. Biediger; P. Douglas Boatman; Mitsuru Shindo; Chase C. Smith; Soekchan Kim; Hossain Nadizadeh; Yukio Suzuki; Chunlin Tao; Phong Vu; Suhan Tang; Pingsheng Zhang; Krishna K. Murthi; Lisa N. Gentile; Jyanwei H. Liu (1994). "First total synthesis of taxol. 1. Functionalization of the B ring". J. Am. Chem. Soc. 116 (4): 1597–1598. doi:10.1021/ja00083a066.
- Robert A. Holton; Hyeong-Baik Kim; Carmen Somoza; Feng Liang; Ronald J. Biediger; P. Douglas Boatman; Mitsuru Shindo; Chase C. Smith; Soekchan Kim; Hossain Nadizadeh; Yukio Suzuki; Chunlin Tao; Phong Vu; Suhan Tang; Pingsheng Zhang; Krishna K. Murthi; Lisa N. Gentile; Jyanwei H. Liu (1994). "First Total Synthesis of Taxol. 2. Completion of the C and D Rings". J. Am. Chem. Soc. 116 (4): 1599–1600. doi:10.1021/ja00083a067.
- Nicolaou, K.C.; Yang, Z.; Liu, J. J.; Ueno, H.; Nantermet, P. G.; Guy, R. K.; Claiborne, C. F.; Renaud, J.; Couladouros, E. A.; Paulvannan, K.; Sorenson, E. J. (1994). "Total synthesis of taxol". Nature. 367 (6464): 630–634. Bibcode:1994Natur.367..630N. doi:10.1038/367630a0. PMID 7906395.
- Paul A. Wender, Neil F. Badham, Simon P. Conway, Paul E. Floreancig, Timothy E. Glass, Christian Gränicher, Jonathan B. Houze, Jan Jänichen, Daesung Lee, Daniel G. Marquess, Paul L. McGrane, Wei Meng, Thomas P. Mucciaro, Michel Mühlebach, Michael G. Natchus, Holger Paulsen, David B. Rawlins, Jeffrey Satkofsky, Anthony J. Shuker, James C. Sutton, Richard E. Taylor, and Katsuhiko Tomooka (1997) "The Pinene Path to Taxanes. 5. Stereocontrolled Synthesis of a Versatile Taxane Precursor" J. Am. Chem. Soc. 119 (11), 2755-2756 (Communication) doi:10.1021/ja9635387
- Paul A. Wender, Neil F. Badham, Simon P. Conway, Paul E. Floreancig, Timothy E. Glass, Jonathan B. Houze, Nancy E. Krauss, Daesung Lee, Daniel G. Marquess, Paul L. McGrane, Wei Meng, Michael G. Natchus, Anthony J. Shuker, James C. Sutton, and Richard E. Taylor (1997) "The Pinene Path to Taxanes. 6. A Concise Stereocontrolled Synthesis of Taxol" J. Am. Chem. Soc. 119 (11), 2757-2758 (Communication) doi:10.1021/ja963539z
- Koichiro Morihira, Ryoma Hara, Shigeru Kawahara, Toshiyuki Nishimori, Nobuhito Nakamura, Hiroyuki Kusama, and Isao Kuwajima (1998) "Enantioselective Total Synthesis of Taxol" J. Am. Chem. Soc. 120 (49), 12980-12981 (Communication) doi:10.1021/ja9824932
- Hiroyuki Kusama, Ryoma Hara, Shigeru Kawahara, Toshiyuki Nishimori, Hajime Kashima, Nobuhito Nakamura, Koichiro Morihira, and Isao Kuwajima (2000) "Enantioselective Total Synthesis of (−)-Taxol" J. Am. Chem. Soc. 122 (16) 3811-3820. doi:10.1021/ja9939439
- Isamu Shiina, Hayato Iwadare, Hiroki Sakoh, Masatoshi Hasegawa, Yu-ichirou Tani, and Teruaki Mukaiyama (1998) "A New Method for the Synthesis of Baccatin III" Chemistry Letters 27 (1), 1-2 doi:10.1246/cl.1998.1
- Takayuki Doi, Shinichiro Fuse, Shigeru Miyamoto, Kazuoki Nakai, Daisuke Sasuga and Takashi Takahashi (2006) "A Formal Total Synthesis of Taxol Aided by an Automated Synthesizer" Chemistry: An Asian Journal 1 (3), 370-383. doi:10.1002/asia.200600156
- Keisuke Fukaya, Yuta Tanaka, Ayako C. Sato, Keisuke Kodama, Hirohisa Yamazaki, Takeru Ishimoto, Yasuyoshi Nozaki, Yuki M. Iwaki, Yohei Yuki, Kentaro Umei, Tomoya Sugai, Yu Yamaguchi, Ami Watanabe, Takeshi Oishi, Takaaki Sato, and Noritaka Chida (2015) "Synthesis of Paclitaxel. 1. Synthesis of the ABC Ring of Paclitaxel by SmI2-Mediated Cyclization" Organic Letters 17 (11), 2570-2573 doi:10.1021/acs.orglett.5b01173
- Keisuke Fukaya, Keisuke Kodama, Yuta Tanaka, Hirohisa Yamazaki, Tomoya Sugai, Yu Yamaguchi, Ami Watanabe, Takeshi Oishi, Takaaki Sato, and Noritaka Chida (2015) "Synthesis of Paclitaxel. 2. Construction of the ABCD Ring and Formal Synthesis" Organic Letters 17 (11), 2574-2577 doi:10.1021/acs.orglett.5b01174
- D. F. Taber (October 5, 2015) The Sato/Chida Synthesis of Paclitaxel Organic Chemistry Highlights (www.organic-chemistry.org)
- Sho Hirai, Masayuki Utsugi, Mitsuhiro Iwamoto, Masahisa Nakada (2015), "Formal Total Synthesis of (−)-Taxol through Pd-Catalyzed Eight-Membered Carbocyclic Ring Formation" Chemistry: A European Journal 21 (1), 355–359. doi:10.1002/chem.201404295
- Yuzuru Kanda, Hugh Nakamura, Shigenobu Umemiya, Ravi Kumar Puthukanoori, Venkata Ramana Murthy Appala, Gopi Krishna Gaddamanugu, Bheema Rao Paraselli, and Phil Baran (2020), "Two-Phase Synthesis of Taxol" doi:10.26434/chemrxiv.12061620.v1
- Changxia Yuan, Yehua Jin, Nathan C. Wilde, Phil S. Baran (2016) "Short, Enantioselective Total Synthesis of Highly Oxidized Taxanes" Angew. Chem. Int. Ed. 55 (29), 8280-8284 doi:10.1002/anie.201602235
- Bruce Ganem and Roland R. Franke (2007) "Paclitaxel from Primary Taxanes: A Perspective on Creative Invention in Organozirconium Chemistry" J. Org. Chem. 72 (11), 3981-3987. doi:10.1021/jo070129s
- MyDoanh Chau, Stefan Jennewein, Kevin Walker, and Rodney Croteau (2004) Taxol Biosynthesis: Molecular Cloning and Characterization of a Cytochrome P450 Taxoid 7β-Hydroxylase Chemistry & Biology, 11 (5), 663-672, doi:10.1016/j.chembiol.2004.02.025
- Ajikumar, Parayil Kumaran; Xiao, Wen-Hai; Tyo, Keith E. J.; Wang, Yong; Simeon, Fritz; Leonard, Effendi; Mucha, Oliver; Phon, Too Heng; Pfeifer, Blaine; Stephanopoulos, Gregory (2010). "Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli". Science. 330 (6000): 70–74. Bibcode:2010Sci...330...70A. doi:10.1126/science.1191652. PMC 3034138. PMID 20929806.
External links
- Paclitaxel Total Syntheses @ SynArchive.com
- Taxolog for Taxol research, founded by Holto
- The complete Taxol story from Chemical & Engineering News: Article
- Extensive Florida State University article
- Story of taxol total synthesis