Padimate O
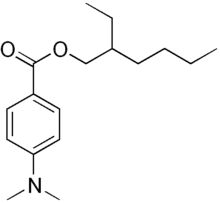
Padimate O is an organic compound related to the water-soluble compound PABA (4-aminobenzoic acid) that is used as an ingredient in some sunscreens. This yellowish water-insoluble oily liquid is an ester formed by the condensation of 2-ethylhexanol with dimethylaminobenzoic acid. Other names for padimate O include 2-ethylhexyl 4-dimethylaminobenzoate, Escalol 507, octyldimethyl PABA, and OD-PABA.
 | |
| Names | |
|---|---|
| IUPAC name
2-ethylhexyl 4-(dimethylamino)benzoate | |
| Other names
2-ethylhexyl dimethyl PABA Escalol 507 Sundown | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.040.248 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H27NO2 | |
| Molar mass | 277.408 g·mol−1 |
| Density | 0.99 g/cm3 |
| Melting point | <25 °C |
| Boiling point | 362 °C (684 °F; 635 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Photobiology
Padimate O absorbs ultraviolet rays, thereby preventing direct DNA damage by UV-B. However, the thus-excited padimate O molecule may then react with DNA to produce indirect DNA damage, similar to the effects of ionizing radiation. An in vitro yeast study conducted in 1993 demonstrated the sunlight-induced mutagenicity of padimate O.[1] The photobiological properties of padimate O resemble those of Michler's ketone, which is considered photocarcinogenic in rats and mice. These findings suggest that padimate O might also be photocarcinogenic.[2]
However, multiple in vivo studies conducted in hairless mice following topical application of padimate O have demonstrated no carcinogenic effects and that padimate O reduces the number of and delays the appearance of UV-induced skin tumors.[3][4][5][6]
See also
- Padimate A, a related sunscreen ingredient
- Sunscreen controversy.
References
- Knowland, John; McKenzie, Edward A; McHugh, Peter J; Cridland, Nigel A (1993). "Sunlight-induced mutagenicity of a common sunscreen ingredient". FEBS Letters. 324 (3): 309–13. doi:10.1016/0014-5793(93)80141-G. PMID 8405372.
- Gulston, Melanie; Knowland, John (1999). "Illumination of human keratinocytes in the presence of the sunscreen ingredient Padimate-O and through an SPF-15 sunscreen reduces direct photodamage to DNA but increases strand breaks". Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 444: 49–60. doi:10.1016/S1383-5718(99)00091-1. PMID 10477339.
- Kligman, Lorraine H.; Akin, Frank J.; Kligman, Albert M. (1980). "Sunscreens prevent ultraviolet photocarcinogenesis". Journal of the American Academy of Dermatology. 3 (1): 30–5. doi:10.1016/S0190-9622(80)80221-0. PMID 6967495.
- Bissett, Donald L.; McBride, James F.; Hannon, Daniel P.; Patrick, Larry F. (1991). "Time-dependent decrease in sunscreen protection against chronic photodamage in UVB-irradiated hairless mouse skin". Journal of Photochemistry and Photobiology B: Biology. 9 (3–4): 323–334. doi:10.1016/1011-1344(91)80169-I.
- Bissett, Donald L; McBride, James F (1996). "Synergistic topical photoprotection by a combination of the iron chelator 2-furildioxime and sunscreen". Journal of the American Academy of Dermatology. 35 (4): 546–9. doi:10.1016/S0190-9622(96)90677-5. PMID 8859281.
- Kerr, Caroline (1998). "The effects of two UVB radiation-absorbing sunscreens on UV radiation-induced carcinogenesis, suppression of the contact hypersensitivity response and histological changes in the hairless mouse". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 422 (1): 161–4. doi:10.1016/S0027-5107(98)00188-2. PMID 9920441.
