Pauson–Khand reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone.[1][2] The reaction was discovered by Ihsan Ullah Khand (1935-1980), who was working as a postdoctoral associate with Peter Ludwig Pauson (1925-2013)[3] at the University of Strathclyde in Glasgow. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient and catalytic.[4][5]
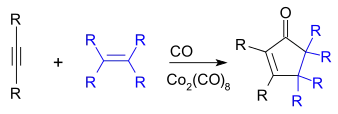
| Pauson–Khand reaction | |
|---|---|
| Named after | Peter Ludwig Pauson Ihsan Ullah Khand |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | pauson-khand-reaction |
| RSC ontology ID | RXNO:0000464 |
With unsymmetrical alkenes or alkynes, regioselectivity can be problematic, but less so with intramolecular reactions.[6]

The reaction works with both terminal and internal alkynes although internal alkynes tend to give lower yields. The order of reactivity for the alkene is strained cyclic alkene > terminal alkene > disubstituted alkene > trisubstituted alkene. Unsuitable alkenes are tetrasubstituted alkenes and alkenes with strongly electron withdrawing groups.[7]
Takayama and co-workers used an intramolecular Pauson–Khand reaction to cyclise an enyne containing a tert-butyldiphenylsilyl (TBDPS) protected primary alcohol.[8] This was a key step in the asymmetric total synthesis of the Lycopodium alkaloid huperzine-Q. The inclusion of a cyclic siloxane moiety in the reagent ensures that the product is formed with the desired conformation[9] – only a single enantiomer of the product was obtained in the Pauson–Khand reaction sequence.[8]

Variations
Wilkinson's catalyst, based on the transition metal rhodium, also effectively catalyses PK reactions but requires silver triflate as a co-catalyst.[10]

Molybdenum hexacarbonyl is a carbon monoxide donor in PK-type reactions between allenes and alkynes with dimethyl sulfoxide in toluene.[11]

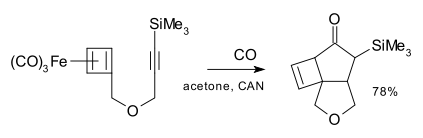
Cyclobutadiene also lends itself to a [2+2+1] cycloaddition although this reactant is generated in situ from decomplexation of stable cyclobutadiene iron tricarbonyl with ceric ammonium nitrate (CAN).[12]
An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, [(CO)2RhCl]2, in the synthesis of (+)-phorbol by Phil Baran. In addition to using a rhodium catalyst, this synthesis features an intramolecular cyclization that results in the normal 5-membered α,β-cyclopentenone as well as 7-membered ring.[13]
See also
References
- Pauson, P. L.; Khand, I. U. (1977). "Uses of Cobalt-Carbonyl Acetylene Complexes in Organic Synthesis". Ann. N. Y. Acad. Sci. 295 (1): 2–14. doi:10.1111/j.1749-6632.1977.tb41819.x.
- Blanco-Urgoiti, Jaime; Añorbe, Loreto; Pérez-Serrano, Leticia; Domínguez, Gema; Pérez-Castells, Javier (2004). "The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules". Chem. Soc. Rev. 33 (1): 32–42. doi:10.1039/b300976a. PMID 14737507.
- Werner, Helmut (2014). "Obituary: Peter Ludwig Pauson (1925–2013)". Angew. Chem. Int. Ed. 53 (13): 3309. doi:10.1002/anie.201400432.
- Schore, Neil E. (1991). "The Pauson–Khand Cycloaddition Reaction for Synthesis of Cyclopentenones". Org. React. 40: 1–90. doi:10.1002/0471264180.or040.01. ISBN 0471264180.
- Gibson, Susan E.; Stevenazzi, Andrea (2003). "The Pauson–Khand Reaction: The Catalytic Age Is Here!". Angew. Chem. Int. Ed. 42 (16): 1800–1810. doi:10.1002/anie.200200547. PMID 12722067.
- Jeong, Nakcheol; Hwang, Sung Hee; Lee, Youngshin; Chung, Young Keun (1994). "Catalytic version of the Intramolecular Pauson-Khand Reaction". Journal of the American Chemical Society. 116 (7): 3159. doi:10.1021/ja00086a070.
- Strategic applications of named reactions in organic synthesis: background and details mechanisms 2007 László Kürti,Barbara Czakó
- Nakayama, Atsushi; Kogure, Noriyuki; Kitajima, Mariko; Takayama, Hiromitsu (2011). "Asymmetric Total Synthesis of a Pentacyclic Lycopodium Alkaloid: Huperzine‐Q". Angew. Chem. Int. Ed. 50 (35): 8025–8028. doi:10.1002/anie.201103550. PMID 21751323.
- Ho, Tse-Lok (2016). "Dicobalt Octacarbonyl". Fiesers' Reagents for Organic Synthesis. 28. John Wiley & Sons. pp. 251–252. ISBN 9781118942819.
- Nakcheol Jeong, Byung Ki Sung, Jin Sung Kim, Soon Bong Park,Sung Deok Seo, Jin Young Shin, Kyu Yeol In, Yoon Kyung Choi Pauson–Khand-type reaction mediated by Rh(I) catalysts Pure Appl. Chem., Vol. 74, No. 1, pp. 85–91, 2002. (Online article)
- Kent, J (1995). "A new allenic Pauson-Khand cycloaddition for the preparation of α-methylene cyclopentenones". Tetrahedron Letters. 36 (14): 2407–2410. doi:10.1016/0040-4039(95)00315-4.
- Intramolecular [2+2+1] Cycloadditions with (Cyclobutadiene)tricarbonyliron Benjamin A. Seigal, Mi Hyun An, Marc L. Snapper Angewandte Chemie International Edition Volume 44, Issue 31 , Pages 4929 - 4932 2005. doi:10.1002/anie.200501100
- Kawamura, Shuhei; Chu, Hang; Felding, Jakob; Baran, Phil S. (2016). "Nineteen-step total synthesis of (+)-phorbol". Nature. 532 (7597): 90–93. Bibcode:2016Natur.532...90K. doi:10.1038/nature17153. PMC 4833603. PMID 27007853.