Penta-graphene
Penta-graphene is a hypothetical carbon allotrope composed entirely of carbon pentagons and resembling the Cairo pentagonal tiling.[2] Penta-graphene was proposed in 2014 on the basis of analyses and simulations.[2] Further calculations showed that it is unstable in its pure form,[3] but can be stabilized by hydrogenation.[1] Owing to its atomic configuration, penta-graphene has an unusually negative Poisson’s ratio and very high ideal strength believed to exceed that of a similar material, graphene.[2]
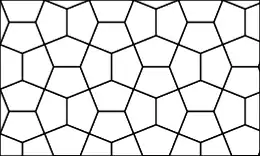
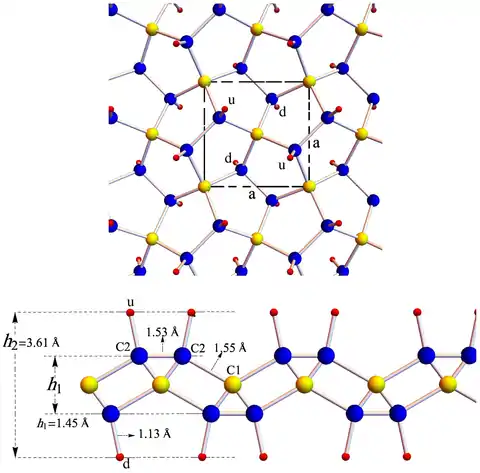
Penta-graphene contains both sp2 and sp3 hybridized carbon atoms. Contrary to graphene, which is a good conductor of electricity, penta-graphene is an insulator with an indirect band gap of 4.1–4.3 eV. Its hydrogenated form is called penta-graphane. It has a diamond-like structure with sp3 and no sp2 bonds, and therefore a wider band gap (ca. 5.8 eV) than penta-graphene.[1] Chiral penta-graphene nanotubes have also been studied as metastable allotropes of carbon.[4][2][5]
References
- Einollahzadeh, Hamideh; Fazeli, Seyed Mahdi; Dariani, Reza Sabet (2016). "Studying the electronic and phononic structure of penta-graphane". Science and Technology of Advanced Materials. 17 (1): 610–617. arXiv:1511.06850. doi:10.1080/14686996.2016.1219970. PMC 5102001. PMID 27877907.
- Zhang, S.; Zhou, J.; Wang, Q.; Chen, X.; Kawazoe, Y.; Jena, P. (2015). "Penta-graphene: A new carbon allotrope". Proceedings of the National Academy of Sciences. 112 (8): 201416591. Bibcode:2015PNAS..112.2372Z. doi:10.1073/pnas.1416591112. PMC 4345574. PMID 25646451.
- Ewels, Christopher P.; Rocquefelte, Xavier; Kroto, Harold W.; Rayson, Mark J.; Briddon, Patrick R.; Heggie, Malcolm I. (2015). "Predicting experimentally stable allotropes: Instability of penta-graphene". Proceedings of the National Academy of Sciences. 112 (51): 15609–12. Bibcode:2015PNAS..11215609E. doi:10.1073/pnas.1520402112. PMC 4697406. PMID 26644554.
- Quijano-Briones, JJ.; Fernandez_escamilla, HN; Tlahuice-Flores, Alfredo. (2017). "Chiral penta-graphene nanotubes: Structure, bonding and electronic properties". Computational and Theoretical Chemistry. 1108: 201520402. doi:10.1016/j.comptc.2017.03.019.
- Avramov, P; Demin, V; Luo, M (2015). "Translation Symmetry Breakdown in Low-Dimensional Lattices of Pentagonal Rings". J. Phys. Chem. Lett. 6 (22): 4525–4531. doi:10.1021/acs.jpclett.5b02309.