Pentaamminechlororhodium dichloride
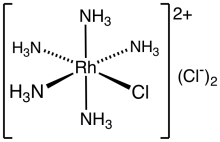
Pentamminechlororhodium dichloride is the dichloride salt of the coordination complex [RhCl(NH3)5]2+. It is a yellow, water-soluble solid. The salt is an intermediate in the purification of rhodium from its ores.
 | |
| Names | |
|---|---|
| Other names
Claus' salt, Pentaamminechlororhodium dichloride, chloropentaamminerhodium dichloride, chloropentaamminerhodium chloride, chloropentamminerhodium(III) dichloride, chloropentamminerhodium(III) chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.082 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| [RhCl(NH3)5]Cl2 | |
| Appearance | yellow solid |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
As shown by X-ray crystallography, the salt consists of the octahedral complex [RhCl(NH3)5]2+ and two chloride counterions.[1] It forms from the reaction of rhodium trichloride and ammonia in ethanol.[2] Two chloride anions are labile, whereas the coordinated chloride ligand is not.
Treatment of [RhCl(NH3)5]Cl2+ with zinc dust in the presence of ammonia gives the hyride complex [RhH(NH3)5]Cl2+.[2][3]
References
- Hambley, Trevor W.; Lay, Peter A. (1986). "Comparisons of pi-Bonding and Hydrogen Bonding in Isomorphous Compounds: [M(NH3)5Cl]Cl2 (M = Cr, Co, Rh, Ir, Ru, Os)". Inorganic Chemistry. 25 (25): 4553–4558. doi:10.1021/ic00245a020.
- Osborn, J. A.; Thomas, K.; Wilkinson, G. (1972). "Pentaamminechlororhodium(III) Dichloride and Pentaamminehydridorhodium(III) Sulfate". Inorganic Syntheses. 13: 213–215. doi:10.1002/9780470132449.ch43.
- Thomas, K.; Osborn, J. A.; Powell, A. R.; Wilkinson, G. (1968). "The Preparation of Hydridopentammine- and Hydridoaquotetramminerhodium(III) Sulphates and Other Salts; the Formation of Alkyl and Fluoroalkyl Derivatives". Journal of the Chemical Society A: 1801. doi:10.1039/j19680001801.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.