Pentazenium
The pentazenium cation (also known as pentanitrogen) is a positively charged polynitrogen ion of the chemical formula N+
5. Together with dinitrogen, solid nitrogen polymers and the azide anion, it is one of only three polynitrogen species obtained in bulk quantities.
 | |
| Names | |
|---|---|
| Other names
Pentanitrogen cation | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| N+ 5 | |
| Molar mass | 70.0335 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Within the High Energy Density Matter research program, run by the U.S. Air Force since 1986, systematic attempts to approach polynitrogen compounds began in 1998, when Air Force Research Laboratory at Edwards AFB became interested in researching alternatives to the highly toxic hydrazine-based rocket fuel and simultaneously funded several such proposals. Karl O. Christe, then, a senior investigator at AFRL, chose to attempt building linear N+
5 out of N
2F+
and N−
3, based on the proposed bond structure:[1]
- [F−N≡N]+ + HN=N=N → [N≡N−N=N=N]+ + HF
The reaction succeeded, and N+
5AsF−
6 was created in sufficient quantities to be fully characterized by NMR, IR and Raman spectroscopy in 1999.[2] The salt was highly explosive, but when AsF
5 was replaced by SbF
5, a stronger Lewis acid, much more stable N+
5SbF−
6 was produced, shock-resistant and thermally stable up to 60–70 °C. This made bulk quantities, easy handling, and X-ray crystal structure analysis possible.[3]
Preparation
Reaction of N
2F+
and HN
3 in dry HF at −78 °C is the only known method so far:
- cis-N
2F
2 + SbF
5 → [N
2F]+
[SbF
6]− - [N
2F]+
[SbF
6]−
+ HN
3 → [N
5]+
[SbF
6]−
+ HF
Chemistry
N+
5 is capable of oxidizing water, NO, NO
2 and Br
2, but not Cl
2 or O
2; its electron affinity is 10.44 eV (1018.4 kJ/mol). For this reason, N+
5 must be prepared and handled in a dry environment:
- 4 N+
5AsF−
6 + 2 H
2O → 4 HF + 4 AsF
5 + 10 N
2 + O
2 - 2 N+
5SbF−
6 + 2 Br
2 → 2 Br+
2SbF−
6 + 5 N
2
Due to stability of the fluoroantimonate, it is used as the precursor for all other known salts, typically accomplished by metathesis reactions in non-aqueous solvents such as HF, SO
2, CHF
3, or CH
3CN, where suitable hexafluoroantimonates are insoluble:
- 2 N+
5SbF−
6 + A+
B−
→ N+
5B−
+ ASbF
6
The most stable salts of N+
5 decompose when heated to 50–60 °C: N+
5SbF−
6, N+
5SbF−
5, and N
5B(CF
3)
4, while the most unstable salts that were obtained and studied, N+
5[P(N
3)−
6] and N+
5[B(N
3)−
4] were extremely shock and temperature sensitive, exploding in solutions as dilute as 0.5 mmol. A number of salts, such as fluoride, azide, nitrate, or perchlorate, cannot be formed.[1]
Structure and bonding
In valence bond theory, pentazenium can be described by six resonance structures:
- [N≡N+−N−−N+≡N]+ ↔ [N−=N+=N−N+≡N]+ ↔ [N≡N+−N=N+=N−]+ ↔ [N≡N+−N+≡N+−N2−]+ ↔ [N2−−N+≡N+−N+≡N]+ ↔ [N−=N+=N+=N+=N−]+,
where the last three pictured have smaller contributions to the overall structure because they have less favorable formal charge states than the first three.[4]
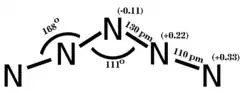
According to both ab initio calculations and the experimental X-ray structure, the cation is planar, symmetric, and approximately V-shaped, with bond angles 111° at the central atom (angle N2–N3–N4) and 168° at the second and fourth atoms (angles N1–N2–N3 and N3–N4–N5). The bond lengths for N1–N2 and N4–N5 are 1.10 Å and the bond lengths N2–N3 and N3–N4 are 1.30 Å.[3]
References
- Christe, Karl O. (14 Jun 2007). "Recent Advances in the Chemistry of N+
5, N−
5 and High-Oxygen Compounds". Propellants, Explosives, Pyrotechnics. 32 (3): 194–204. doi:10.1002/prep.200700020. - Christe, Karl O.; William W. Wilson; Jeffrey A. Sheehy; Jerry A. Boatz (12 Jul 1999). "N+
5: A Novel Homoleptic Polynitrogen Ion as a High Energy Density Material". Angewandte Chemie International Edition. 38 (13–14): 2004–2009. doi:10.1002/(SICI)1521-3773(19990712)38:13/14<2004::AID-ANIE2004>3.0.CO;2-7. - Vij, Ashwani; William W. Wilson; Vandana Vij; Fook S. Tham; Jeffrey A. Sheehy; Karl O. Christe (9 Jun 2001). "Polynitrogen Chemistry. Synthesis, Characterization, and Crystal Structure of Surprisingly Stable Fluoroantimonate Salts of N+
5". J. Am. Chem. Soc. 123 (26): 6308–6313. doi:10.1021/ja010141g. PMID 11427055. - "Method of drawing the Lewis Structures of N5+". Chemistry Net Blogspot. Blogger. October 31, 2012. Retrieved November 8, 2016.
