Solid nitrogen
Solid nitrogen is the solid form of the element nitrogen. It is an important component of the surfaces of Pluto[1] and outer moons of the Solar System such as Neptune's Triton.[2] Under low or moderate pressure solid nitrogen contains dinitrogen molecules held together by London dispersion forces.[3] At standard atmospheric pressure for Earth, this solid melts at 63.23 K,[4] but this is not true at other pressures. Non-molecular forms of solid nitrogen produced by extreme pressures have a higher energy density than any other non-nuclear material.[5]
.png.webp)
Solid nitrogen was first made in 1884, by first liquefying hydrogen with evaporating liquid nitrogen, and then allowing the liquid hydrogen to freeze the nitrogen.[6] Karol Olszewski achieved a world record lowest temperature in 1884 by evaporating vapour from solid nitrogen getting down to 48 K.[7] Solid nitrogen is normally made in a laboratory by evaporating liquid nitrogen in a vacuum. The solid produced is porous.[8]
Bulk properties
Solid nitrogen has several properties relevant to its formation of rocks in the outer Solar System. Even at the low temperatures of solid nitrogen it is fairly volatile and can sublime to form an atmosphere, or condense back into nitrogen frost. At 58 K the ultimate compressive strength is 0.24 MPa. Strength increases as temperature lowers becoming 0.54 MPa at 40.6 K. Elastic modulus varies from 161 to 225 MPa over the same range.[9] Compared to other materials, solid nitrogen loses cohesion at low pressures and flows in the form of glaciers when amassed. Yet its density is higher than that of water ice, so the forces of buoyancy will naturally transport blocks of water ice towards the surface. This effect has been most clearly observed on Pluto (by the New Horizons space probe in 2015), where water ice makes up a major part of the surface layers as icebergs on top of nitrogen ice.[10]
Solid nitrogen mixes with solid carbon monoxide and methane on the surface of Pluto.[10]
The thermal conductivity of solid nitrogen is 0.7 W m−1 K−1.[11] Thermal conductivity varies with temperature and the relation is given by k = 180.2×T0.1041 watts per kelvin.[12] Specific heat is given by 926.91×e0.0093T joules per kilogram per kelvin.[12] Its appearance at 50 K is transparent, while at 20 K it is white.
Nitrogen frost has a density of 0.85 g cm−3.[13] As a bulk material the crystals are pressed together and density is near that of water. It is temperature dependent and given by ρ = 0.0134T2 − 0.6981T + 1038.1 kg/m3.[12] The volume coefficient of expansion is given by 2×10−6T2 − 0.0002T + 0.006 K−1.[12]
The index of refraction at 6328 Å is 1.25 and hardly varies with temperature.[13]
The speed of sound in solid nitrogen is 1452 m/s at 20 K and 1222 m/s at 44 K. The longitudinal velocity ranges from 1850 m/s at 5 K to 1700 m/s at 35 K. With temperature rise the nitrogen changes phase and the longitudinal velocity drops rapidly over a small temperature range to below 1600 m/s and then it slowly drops to 1400 m/s near the melting point. The transverse velocity is much lower ranging from 900 to 800 m/s over the same temperature range.[3]
The bulk modulus of s-N2 is 2.16 GPa at 20 K, and 1.47 GPa at 44 K.[3] At temperatures below 30 K solid nitrogen will undergo brittle failure, particularly if strain is applied quickly. Above this temperature the failure mode is ductile failure. Dropping 10 K makes the solid nitrogen 10 times as stiff.[3]
Melting
At standard atmospheric pressure, the melting point of N2 is 63.23 K.[4] Solid nitrogen melts at a higher temperature with increasing ambient pressure.[14] The slope of the melting point line of the phase diagram is 190 K GPa−1.[14] At 2.8 GPa nitrogen melts at 308 K, at 4 GPa it melts at 368 K, and at 7 GPa it melts at 484 K.[14] The melting point increases all the way to 1920 K at a pressure of 50 GPa. Above this pressure the melting point decreases. This is due to a change in the liquid, which becomes denser than the solid at that pressure. The liquid is predicted to become a polymer. The melting point drops to 1400 K at 71 GPa.[15]
Solid nitrogen is slightly soluble in liquid hydrogen. At 15K there are somewhere between 1010 and 1011 molecules of nitrogen per cm3 of liquid hydrogen.[16] At the boiling point of hydrogen the amount in solution is 10−8 molar fraction.[17] At 32.5 K the molar concentration of dissolved N2 in close to critical H2 is 7.0×10−6.[17]
Sublimation
When the pressure is below the triple point, solid nitrogen directly sublimes to gas. The triple point is at 63.14±0.06 K and 0.1255±0.0005 bar.[18] The vapour pressure has been measured from 20 K up to the triple point. For α-nitrogen (below 35 K) the logarithm of the pressure is given by 12.40 −807.4 × T−1 −3926 T−2 +6.297×10+ 4T−3 −4.633× 10 +5T−4 1.325× 10+ 6T−5. For β-nitrogen it is given by 8.514 −458.4T−1 −19870 T−2 4.800 × 10+ 5T−3 −4.524 × 10+6T−4.[18] Where the solid is not pure nitrogen, the vapour pressure can be estimated using Raoult's law in which the pressure is reduced by the molar concentration. This calculation is relevant for the atmosphere of outer solar system bodies, where there could be a 1% contamination with carbon monoxide and methane.[18]
Crystal structure
β
There are several known solid forms of molecular dinitrogen. At ambient pressures there are two solid forms. β-N2 is a hexagonal close packed structure which exists from 35.6 K up to 63.15 K at which point it melts.[14] (63.15 K is exactly -210 °C, so it may be rounded and the correct melting point may be 63.23 K.[4]) At 45 K the unit cell has a=4.050 Å and c=6.604 Å.[14] At 4125 atmospheres pressure and 49 K, the unit cell sizes have shrunk to a=3.861 Å c=6.265 Å.[19] If the pressure is increased the c/a ratio stays the same.[19]
In the β phase, the molecule centres are hexagonal close packed. This means that the c/a ratio is ≈ 1.633 = √8/3. The nitrogen molecules are randomly tipped at an angle of 55° from the c-axis. There is a strong quadrupole-quadrupole interaction between the molecules.[19]
α
Another phase termed α-N2 exists below 35.6 K at low pressure and has a cubic structure. The space group is Pa3. At 21 K the unit cell dimension is 5.667 Å.[14] Under 3785 bars this reduces to 5.433 Å.[19] At low temperatures the α-phase can be compressed to 3500 atmospheres before it changes (to γ), and as the temperature rises above 20 K, this pressure rises to about 4500 atmospheres.[19]
The nitrogen molecules are located on the body diagonals of the unit cell cube.[19]
γ
The tetragonal γ form exists at low temperatures below 44.5 K between about 0.3 GPa and 3 GPa pressure.[14] The triple point for α/β/γ2 is at 0.47 GPa and 44.5 K.[14] The space group of the γ phase is P42/mnm and its unit cell has lattice constants a=3.957 Å, c=5.109 Å at 20 K and 4000 bar.[14] The 15N isotope converts at a pressure 400 atmospheres lower to the γ form than natural nitrogen, at 20 K.[19]
In the γ form, nitrogen molecules appear to have the shape of a prolate spheroid, 4.34 Å in the long dimension, and 3.39 Å in the short diameter. The boundary of the molecule appears at an electron density of 0.0135 eÅ−3. The molecules line up in rows end to end diagonally on the ab plane. These rows stack side by side with molecules offset by half their length to form layers in the (001) plane, perpendicular to the c-axis. Layers stack on top of each other each rotated by 90° compared to the plane below. Coordinates of atoms in the unit cell are given by (x,x,0),(-x,-x,0),(1/2+x,1/2-x,1/2),(1/2-x,1/2+x,1/2) with x=r/a√8 and r=interatomic distance in nitrogen molecule, = 1.10 Å. (unit cell dimension as above a=3.957 Å). The molecules can vibrate up to 10° on the ab plane, and up to 15° in the direction of the c axis.[19]
δ
δ-N2 has a triple point with β and γ Nitrogen at 2.3 GPa and 150 K. δ-N2 has a cubic structure with space group pm3n and eight molecules per unit cell. The lattice constant is 6.164 at 300 K and 4.9 GPa.[20] This strucure is the same as for dioxygen (γ-O2) at 50 K. At room temperature and high pressure δ-nitrogen is ordered in its molecular orientation[21]
Above the pressure of 2 GPa there is the lower temperature rhombohedral phase ε-N2 and above 80 K cubic δ-N2.[14] The triple point of δ-N2, β-N2 and liquid is somewhere between 8 and 10 GPa and 555 and 578 K.[14]
ε
ε-N2 is rhombohedral with space group R3c is a high pressure form of dinitrogen, stable at 13 GPa.[22] Cell dimensions are a=8.02 Å, b=8.02 Å, c=11.104 Å, α=β=90°, γ=120°, volume 618.5 Å3, Z=24.[23] ε-nitrogen has disordered orientation.[21]
In the phase diagram ε-N2 appears at pressures above 2 GPa at temperatures below 50 K. Below this the γ form is stable. When heated ε-N2 transforms to δ-N2[24]
ζ
Above 69 GPa ε-N2 transforms to an orthorhombic phase designated by ζ-N2 with a 6% reduction in volume. The space group of ζ-N2 is P2221. The lattice constants are a=4.159 Å, b=2.765 Å, c=5.039 Å with eight atoms per unit cell.[5] At 80 GPa the distance between nitrogen atoms in the molecules is 0.982 Å, but the closest distance to other nitrogen atoms is 1.93 Å. As the pressure increases to 138 GPa the bond in the molecules actually lengthens to 1.002 Å while intermolecular distances shorten.[5]
θ
A ζ-N2 phase compressed to 95 GPa and then heated to over 600 K produces a new structure called θ nitrogen which has a uniform translucent appearance.[25]
ι
ι-N2 can be accessed[26] by isobaric heating of ε-N2 to 750 K at 65 GPa, or, by isothermal decompression of θ-N2 to 69 GPa at 850 K.
The ι-N2 crystal structure[27] is characterised by primitive monoclinic lattice with unit-cell dimensions of: a=9.899(2), b=8.863(2), c=8.726(2) Å, β=91.64(3)° and V=765.2(3) Å3 at 56 GPa and ambient temperature. The space group is P21/c and the unit cell contains 48 N2 molecules arranged into a layered structure.
μ
When the ζ-N2 phase is compressed at room temperature over 150 GPa an amorphous form is produced.[5] This is designated as the μ-phase. It is a narrow gap semiconductor. The μ-phase has been brought to atmospheric pressure by first cooling it to 100 K.[28]
η
η-N is a semiconducting amorphous form of nitrogen. It is formed at pressures between 80 and 270 GPa, and temperatures 10 to 510K. In reflected light it appears black, but does transmit some red or yellow light. In the infrared there is an absorption band around 1700 cm−1. It does not contain N2 molecules. Under even higher pressure of 280 Gpa, it transforms into a metal.[29]
Cubic gauche
Under pressures higher than 110 GPa and temperatures around 2000 K nitrogen forms a network solid, bound by single covalent bonds in what is called a cubic-gauche structure, abbreviated as cg-N. This substance is very stiff with a bulk modulus around 298 GPa, similar to diamond.[30] It is very high-energy.[31] The cubic-gauche form has space group I213.[22] The unit cell edge is 3.805 Å.[22] There are eight nitrogen atoms per unit cell.[22] The bond angles are very close to tetrahedral. The structure contains rings of nitrogen atoms that are fused together. The position of the lone pairs of electrons is ranged so that their overlap is minimised.[28] The difference in bond energy varies from 0.83 eV per atom in nitrogen gas to 4.94 eV per atom, so having a difference in energy of over 4 eV per atom. This cubic-gauche nitrogen is the highest energy non-nuclear material and is being investigated for use in explosives and rocket fuel.[5] Estimates of its energy density vary: a value of 33 kJ g−1 is predicted, which is over three times the energy density of HMX.[32] A more recent predicted value is 10.22 kJ g−1 which, while much lower, is still more than 60% more than HMX.[33] cg-N has all bonds the same length[5] of 1.346 Å at 115 GPa.[30] The cubic-gauche structure for nitrogen is predicted[34] to have bond lengths of 1.40 Å, bond angles of 114.0° and dihedral angles of −106.8°. The term gauche refers to the odd dihedral angles, if it were 0° it would be called cis, and if 180° it would be called trans. The dihedral angle Φ is related to the bond angle θ by sec(Φ) = sec(θ) − 1. The coordinate of one atom in the unit cell at x,x,x also determines the bond angle by cos(θ) = x(x-1/4)/(x2+(x-1/4)2).[34]
Poly-N
Another network solid nitrogen called poly-N and abbreviated pN was predicted in 2006.[22] pN has space group C2/c and cell dimensions a = 5.49 Å, β = 87.68°. Other higher pressure polymeric forms are predicted in theory, and a metallic form is expected if the pressure is high enough.[35]
Black phosphorus nitrogen
When compressing nitrogen to pressures between 120 and 180 GPa and temperatures above 4000 °C,[36][37] it adopts a crystal structure identical to that of black phosphorus (orthorhombic, Cmce space group), hence coined as black phosphorus nitrogen (bp-N) or simply black nitrogen.[38] Like black phosphorus, it is an electrical conductor.[39] The formation of the bp-N structure brings nitrogen in line with heavier pnictogen elements, and reaffirms the trend that elements at high pressure adopt the same structures as the same-group elements below them in the periodic table at lower pressures.[40]
Hexagonal layered polymeric nitrogen
Hexagonal layered polymeric nitrogen (HLP-N) is the third form of polymeric nitrogen found stable under pressure and was experimentally synthesized at 244 GPa and 3300 K. It adopts a tetragonal unit cell (P42bc) in which the single-bonded nitrogen atoms form two layers of interconnected N6 hexagons. It was found to be metastable down to at least 66 GPa.[41]
Linear N8
.png.webp)
Simulations predicted a molecular solid made of N8 (N≡N+-N−-N=N-N−-N+≡N) which is stable at low temperatures and pressures (< 20 GPa).[42] In 2018 experiments support the prediction and show a transformation of hydrazinium azide to molecular N8.[43] The reported N8 decomposes to the ε-N2 below 25 GPa but a remainder of N8 can be at pressure as low as 3 GPa.
Linear N6
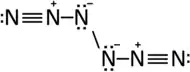
A similar molecule has been predicted by Grechner et al. 2016 to exist at at ambient conditions.[44]
Others
Yet other phases of solid dinitrogen are termed ζ'-N2 and κ-N2.[28]
Related substances
Under pressure nitrogen can form crystalline van der Waals compounds with other molecules. It can form an orthorhombic phase with methane above 5 GPa.[45] With helium He(N2)11 is formed.[21] N2 crystallizes with water in nitrogen clathrate and in a mixture with oxygen O2 and water in air clathrate.[46]
Helium
Solid nitrogen can dissolve 2 mole % helium under pressure in its disordered phases such as the γ-phase. Under higher pressure 9 mol% hHelium, He can react with ε-nitrogen to form a hexagonal birefringent crystalline van der Waals compound. The unit cell contains 22 nitrogen atoms and 2 helium atoms. It has a volume of 580 Å3 for a pressure of 11 GPa decreasing to 515 Å3 at 14 GPa.[21] It resembles the ε-phase.[47] At 14.5 GPa and 295 K the unit cell has space group P63/m and a=7.936 Å c=9.360 Å. At 28 GPa a transition happens in which the orientation of N2 molecules becomes more ordered. When the pressure on He(N2)11 exceeds 135 GPa the substance changes from clear to black, and takes on an amorphous form similar to η-N2.[48]
Methane
Solid nitrogen can crystallise with some solid methane included. At 55 K the molar percentage can range up to 16.35% CH4, and at 40 K only 5%. In the complementary situation, solid methane can include some nitrogen in its crystals, up to 17.31% nitrogen. As the temperature drops, less methane can dissolve in solid nitrogen, and in α-N2 there is a major drop in methane solubility. These mixtures are prevalent in outer Solar System objects such as Pluto that have both nitrogen and methane on their surfaces.[49] At room temperature there is a clathrate of methane and nitrogen in 1:1 ratio formed at pressures over 5.6 GPa.[50]
Carbon monoxide
The carbon monoxide molecule (CO) is very similar to dinitrogen in size, and it can mix in all proportions with solid nitrogen without changing crystal structure. Carbon monoxide is also found on the surfaces of Pluto and Triton at levels below 1%. Variations in the infrared linewidth of carbon monoxide absorption can reveal the concentration.[51]
Noble gases
Neon or xenon atoms can also be included in solid nitrogen in the β and δ phases. Inclusion of neon pushes the β−δ phase boundary to higher pressures.[52] Argon is also very miscible in solid nitrogen.[52] For compositions of argon and nitrogen with 60% to 70% nitrogen, the hexagonal form remains stable to 0 K.[53] A van der Waals compound of xenon and nitrogen exists above 5.3 GPa.[52] A van der Waals compound of neon and nitrogen was shown using Raman spectroscopy.[52] The compound has formula (N2)6Ne7. It has a hexagonal structure, with a=14.400 c=8.0940 at a pressure of 8 GPa. A van der Waals compound with argon is not known.[54]
Hydrogen
With dideuterium, a clathrate (N2)12D2 exits around 70 GPa.[55]
Reactions
Radiation treatment
When solid nitrogen is irradiated by high speed protons or electrons, several reactive radicals are formed, including atomic nitrogen (N), nitrogen cations (N+), dinitrogen cation (N2+), trinitrogen radicals (N3 and N3+), and azide (N3−).[57]
Use
Solid nitrogen is used in a slush mixture with liquid nitrogen in order to cool faster than with liquid nitrogen alone, useful for applications such as sperm cryopreservation.[58] The semi-solid mixture can also be called slush nitrogen[59] or SN2.[60]
Solid nitrogen is used as a matrix on which to store and study reactive chemical species, such as free radicals or isolated atoms.[61] One use is to study dinitrogen complexes of metals in isolation from other molecules.[62]
Natural occurrence
.jpg.webp)
Solid nitrogen forms a large part of the surface of Pluto and the Neptunian moon Triton. On Pluto it was directly observed for the first time in July 2015 by the New Horizons space probe and on Triton it was directly observed by the Voyager 2 space probe in August 1989.
On Triton, solid nitrogen takes the form of frost crystals and a transparent sheet layer of annealed nitrogen ice, often referred to as a "glaze".[2] Geysers of nitrogen gas were observed by Voyager 2 to spew from the subpolar regions around Triton's southern polar ice cap.[63] A possible explanation of this observed phenomenon is that the sun shines through the transparent layer of nitrogen ice, heating the layers beneath. Nitrogen sublimes and eventually erupts through holes in the upper layer, carrying dust along with it and creating dark streaks.
References
- "Flowing nitrogen ice glaciers seen on surface of Pluto after New Horizons flyby". ABC. 25 July 2015. Retrieved 6 October 2015.
- McKinnon, William B.; Kirk, Randolph L. (2014). "Triton". In Spohn, Tilman; Breuer, Doris; Johnson, Torrence (eds.). Encyclopedia of the Solar System (3rd ed.). Amsterdam; Boston: Elsevier. pp. 861–882. ISBN 978-0-12-416034-7.
- Yamashita, Yasuyuki; Kato, Manabu; Arakawa, Masahiko (June 2010). "Experimental study on the rheological properties of polycrystalline solid nitrogen and methane: Implications for tectonic processes on Triton". Icarus. 207 (2): 972–977. Bibcode:2010Icar..207..972Y. doi:10.1016/j.icarus.2009.11.032.
- Lide, David R. (1990–1991). CRC Handbook of Physics and Chemistry (71st ed.). Boca Raton, Ann Arbor, Boston: CRC Press, inc. pp. 4–22 (one page).
- Eremets, M. I.; Gavriliuk, A. G.; Serebryanaya, N. R.; Trojan, I. A.; Dzivenko, D. A.; Boehler, R.; Mao, H. K.; Hemley, R. J. (2004). "Structural transformation of molecular nitrogen to a single-bonded atomic state at high pressures". The Journal of Chemical Physics. 121 (22): 11296–300. Bibcode:2004JChPh.12111296E. doi:10.1063/1.1814074. PMID 15634085. S2CID 25122837.
- Olszewski, K (1884). "Nouveaux essais de liquéfaction de l'hydrogène. Solidification et pression critique de l'azote". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences (in French). 98: 913–915.
- Cohen, E. G. D. (1 January 1977). "Toward Absolute Zero: During the past three centuries attempts to approach the absolute zero of temperature have led to the discovery of many important phenomena, including superconductivity and superfluidity". American Scientist. 65 (6): 752–758. Bibcode:1977AmSci..65..752C. JSTOR 27848176.
- Mikhal'chenko, R. S.; Getmanets, V. F.; Arkhipov, V. T. (September 1972). "Peculiarities of heat transfer in porous solid nitrogen". Journal of Engineering Physics. 23 (3): 1075–1081. Bibcode:1972JEP....23.1075M. doi:10.1007/BF00832213. S2CID 121585322.
- Pederson, R. C.; Miller, C. D.; Arvidson, J. M.; Blount, K.; Schulze, M. (1998). "Problems Involved in Determining the Mechanical Properties of Solid Nitrogen and a Composite of Solid Nitrogen and Aluminum Foam (40 K – 61 K)". In Balachandran, U. B.; Gubser, D. G.; Hartwig, K. T.; Reed, R.; Warnes, W. H.; Bardos, V. A. (eds.). Advances in Cryogenic Engineering (Materials). 44. Springer Science & Business Media. pp. 339–347. ISBN 9781475790566.
- "Pluto's mysterious floating hills". NASA. 2016-02-04. Retrieved 1 May 2016.
- Cook, T.; Davey, G. (June 1976). "The density and thermal conductivity of solid nitrogen and carbon dioxide". Cryogenics. 16 (6): 363–369. Bibcode:1976Cryo...16..363C. doi:10.1016/0011-2275(76)90217-4.
- Trowbridge, A. J.; Melosh, H. J.; Steckloff, J. K.; Freed, A. M. (1 June 2016). "Vigorous convection as the explanation for Pluto's polygonal terrain". Nature. 534 (7605): 79–81. Bibcode:2016Natur.534...79T. doi:10.1038/nature18016. PMID 27251278. Methods section
- Satorre, M. A.; Domingo, M.; Luna, R.; Santonja, C. (30 November 2004). "Density of Methane and Nitrogen at Different Temperatures" (PDF). Springer. Retrieved 1 October 2015.
- Tonkov, E. Yu; Ponyatovsky, E.G. (15 November 2004). Phase Transformations of Elements Under High Pressure. CRC Press. pp. 126–132. ISBN 978-0-8493-3367-5.
- Mukherjee, Goutam Dev; Boehler, Reinhard (30 November 2007). "High-Pressure Melting Curve of Nitrogen and the Liquid-Liquid Phase Transition". Physical Review Letters. 99 (22): 225701. Bibcode:2007PhRvL..99v5701M. doi:10.1103/PhysRevLett.99.225701. PMID 18233298.
- Seidel, G. M.; Maris, H. J.; Williams, F. I. B.; Cardon, J. G. (2 June 1986). "Supercooling of Liquid Hydrogen". Physical Review Letters. 56 (22): 2380–2382. Bibcode:1986PhRvL..56.2380S. doi:10.1103/PhysRevLett.56.2380. PMID 10032971.
- Omar, M.H.; Dokoupil, Z. (May 1962). "Solubility of nitrogen and oxygen in liquid hydrogen at temperatures between 27 and 33°K". Physica. 28 (5): 461–471. Bibcode:1962Phy....28..461O. doi:10.1016/0031-8914(62)90033-2.
- Fray, N.; Schmitt, B. (December 2009). "Sublimation of ices of astrophysical interest: A bibliographic review". Planetary and Space Science. 57 (14–15): 2053–2080. Bibcode:2009P&SS...57.2053F. doi:10.1016/j.pss.2009.09.011.
- Schuch, A. F.; Mills, R. L. (1970). "Crystal Structures of the Three Modifications of Nitrogen 14 and Nitrogen 15 at High Pressure". The Journal of Chemical Physics. 52 (12): 6000–6008. Bibcode:1970JChPh..52.6000S. doi:10.1063/1.1672899.
- Cromer, D. T.; Mills, R. L.; Schiferi, D.; Schwalbe, L. A. (15 January 1981). "The structure of N2 at 49 kbar and 299 K". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 37 (1): 8–11. doi:10.1107/S0567740881002070.
- Vos, W. L.; Finger, L. W.; Hemley, R. J.; Hu, J. Z.; Mao, H. K.; Schouten, J. A. (2 July 1992). "A high-pressure van der Waals compound in solid nitrogen-helium mixtures". Nature. 358 (6381): 46–48. Bibcode:1992Natur.358...46V. doi:10.1038/358046a0. S2CID 4313676.
- Kotakoski, J.; Albe, K. (10 April 2008). "First-principles calculations on solid nitrogen: A comparative study of high-pressure phases". Physical Review B. 77 (14): 144109. Bibcode:2008PhRvB..77n4109K. doi:10.1103/PhysRevB.77.144109.
- NIMS. "Atom Work Materials Database". Retrieved 3 October 2015.
- Mills, R. L.; Olinger, Bart; Cromer, D. T. (1986). "Structures and phase diagrams of N2 and CO to 13 GPa by x-ray diffraction". The Journal of Chemical Physics. 84 (5): 2837. Bibcode:1986JChPh..84.2837M. doi:10.1063/1.450310.
- Goncharov, A.; Gregoryanz, E. (15 April 2004). "Solid Nitrogen at Extreme Conditions of High Pressure and Temperature" (PDF). Lawrence Livermore National Laboratory. Retrieved 5 October 2015.
- Gregoryanz, E.; Goncharov, A. F.; Hemley, R. J.; Mao, H. K.; Somayazulu, M.; Shen, G. (13 December 2002). "Raman, infrared, and x-ray evidence for new phases of nitrogen at high pressures and temperatures". Phys. Rev. B. 66 (22): 224108. Bibcode:2002PhRvB..66v4108G. doi:10.1103/physrevb.66.224108.
- Turnbull, R.; Hanfland, M.; Binns, J.; Martinez-Canales, M.; Frost, M.; Marqués, M.; Howie, R.; Gregoryanz, E. (9 November 2018). "Unusually complex phase of dense nitrogen at extreme conditions". Nature Communications. 9 (1): 4717. Bibcode:2018NatCo...9.4717T. doi:10.1038/s41467-018-07074-4. PMC 6226474. PMID 30413685.
- Plašienka, Dušan; Martoňák, Roman (7 March 2015). "Transformation pathways in high-pressure solid nitrogen: From molecular N2 to polymeric cg-N". The Journal of Chemical Physics. 142 (9): 094505. arXiv:1412.1246. Bibcode:2015JChPh.142i4505P. doi:10.1063/1.4908161. PMID 25747092.
- Gregoryanz, Eugene; Goncharov, Alexander F.; Hemley, Russell J.; Mao, Ho-kwang (13 July 2001). "High-pressure amorphous nitrogen". Physical Review B. 64 (5): 052103. arXiv:cond-mat/0105101v1. doi:10.1103/PhysRevB.64.052103.
- Boehler, Reinhard (November 2005). "Diamond cells and new materials". Materials Today. 8 (11): 34–42. doi:10.1016/S1369-7021(05)71158-5.
- Eremets, Mikhail I.; Gavriliuk, Alexander G.; Trojan, Ivan A.; Dzivenko, Dymitro A.; Boehler, Reinhard (4 July 2004). "Single-bonded cubic form of nitrogen". Nature Materials. 3 (8): 558–563. Bibcode:2004NatMa...3..558E. doi:10.1038/nmat1146. PMID 15235595. S2CID 38483662.
- Yoo, Choong-Shik (February 2003). "Novel Functional Extended Solids at Extreme Conditions". DTIC. p. 11. Retrieved 5 October 2015.
- Bondarchuk, Sergey V.; Minaev, Boris F. (2017). "Super high-energy density single-bonded trigonal nitrogen allotrope—a chemical twin of the cubic gauche form of nitrogen". Physical Chemistry Chemical Physics (9) – via The Royal Society of Chemistry.
- Mailhiot, C.; Yang, L. H.; McMahan, A. K. (1 December 1992). "Polymeric nitrogen". Physical Review B. 46 (22): 14419–14435. Bibcode:1992PhRvB..4614419M. doi:10.1103/PhysRevB.46.14419. PMID 10003540.
- Ma, Yanming; Oganov, Artem R.; Li, Zhenwei; Xie, Yu; Kotakoski, Jani (9 February 2009). "Novel High Pressure Structures of Polymeric Nitrogen". Physical Review Letters. 102 (6): 065501. Bibcode:2009PhRvL.102f5501M. doi:10.1103/PhysRevLett.102.065501. PMID 19257600.
- "Never-before-seen "black nitrogen" plugs puzzle in periodic table". New Atlas. 2 June 2020. Retrieved 16 July 2020.
- “Black Nitrogen” – Scientists Solve a Puzzle of the Periodic Table. On: SciTechDaily. June 6, 2020
- Laniel, Dominique; Winkler, Bjoern; Fedotenko, Timofey; Pakhomova, Anna; Chariton, Stella; Milman, Victor; Prakapenka, Vitali; Dubrovinsky, Leonid; Dubrovinskaia, Natalia (2020-05-28). "High-Pressure Polymeric Nitrogen Allotrope with the Black Phosphorus Structure". Physical Review Letters. 124 (21): 216001. arXiv:2003.02758. doi:10.1103/PhysRevLett.124.216001. ISSN 0031-9007. PMID 32530671. S2CID 212414928.
- Delbert, Caroline (4 June 2020). "Scientists Have Created Black Nitrogen". Popular Mechanics. Retrieved 16 July 2020.
- Ultrahigh-pressure mineralogy : physics and chemistry of the earth's deep interior. Hemley, Russell J. (Russell Julian). Washington, DC: Mineralogical Society of America. 1998. ISBN 0-939950-48-0. OCLC 40542380.CS1 maint: others (link)
- Laniel, D.; Geneste, G.; Weck, G.; Mezouar, M.; Loubeyre, P. (2019-02-11). "Hexagonal Layered Polymeric Nitrogen Phase Synthesized near 250 GPa". Physical Review Letters. 122 (6): 066001. Bibcode:2019PhRvL.122f6001L. doi:10.1103/PhysRevLett.122.066001. ISSN 0031-9007. PMID 30822079.
- Hirshberg, Barak; Krylov, Anna I.; Gerber, R. Benny (January 2014). "Calculations predict a stable molecular crystal of N8" (PDF). Nature Chemistry. 6 (1): 52–56. Bibcode:2014NatCh...6...52H. doi:10.1038/nchem.1818. ISSN 1755-4349. PMID 24345947.
- Duwal, Sakun; Ryu, Young-Jay; Kim, Minseob; Yoo, Choong-Shik; Bang, Sora; Kim, Kyungtae; Hur, Nam Hwi (2018-04-07). "Transformation of hydrazinium azide to molecular N8 at 40 GPa". The Journal of Chemical Physics. 148 (13): 134310. Bibcode:2018JChPh.148m4310D. doi:10.1063/1.5021976. ISSN 0021-9606. OSTI 1432864. PMID 29626901.
- Michael J. Greschner et al. (April 2016). "A New Allotrope of Nitrogen as High-Energy Density Material". The Journal of Physical Chemistry A 120(18). doi:10.1021/acs.jpca.6b01655.
- Aldous, Catherine; Desgreniers, Serge (2008). "Novel van der Waals Solid Phases in the Methane-Nitrogen Binary System" (PDF). Retrieved 21 September 2015.
- Choukroun, Mathieu; Kieffer, Susan W.; Lu, Xinli; Tobie, Gabriel (2013). "Clathrate Hydrates: Implications for Exchange Processes in the Outer Solar System". The Science of Solar System Ices. pp. 409–454. doi:10.1007/978-1-4614-3076-6_12. ISBN 978-1-4614-3075-9.
- Olijnyk, H; Jephcoat, A P (15 December 1997). "High-pressure Raman studies of a nitrogen – helium mixture up to 40 GPa". Journal of Physics: Condensed Matter. 9 (50): 11219–11226. Bibcode:1997JPCM....911219O. doi:10.1088/0953-8984/9/50/022.
- Ninet, S. (1 January 2011). "Structural and vibrational properties of the van der Waals compound (N2)11He up to 135 GPa". Physical Review B. 83 (13): 134107. Bibcode:2011PhRvB..83m4107N. doi:10.1103/PhysRevB.83.134107.
- Protopapa, S.; Grundy, W.M.; Tegler, S.C.; Bergonio, J.M. (June 2015). "Absorption coefficients of the methane–nitrogen binary ice system: Implications for Pluto". Icarus. 253: 179–188. arXiv:1503.00703. Bibcode:2015Icar..253..179P. doi:10.1016/j.icarus.2015.02.027. S2CID 96796422.
- Aldous, Catherine. "Novel van der Waals Solid Phases in the Methane-Nitrogen Binary System" (PDF). www.lightsource.ca. Retrieved 22 September 2015.
- Quirico, Eric; Schmitt, Bernard (July 1997). "A Spectroscopic Study of CO Diluted in N2Ice: Applications for Triton and Pluto". Icarus. 128 (1): 181–188. Bibcode:1997Icar..128..181Q. doi:10.1006/icar.1997.5710.
- Kooi, M. E.; Schouten, J. A. (1 November 1999). "High-pressure Raman investigation of mutual solubility and compound formation in Xe-N2 and NeN2" (PDF). Physical Review B. 60 (18): 12635–12643. Bibcode:1999PhRvB..6012635K. doi:10.1103/PhysRevB.60.12635.
- Nosé, Shuichi; Klein, Michael L. (October 1985). "Molecular dynamics study of the alloy (N2)67(Ar)29". Canadian Journal of Physics. 63 (10): 1270–1273. Bibcode:1985CaJPh..63.1270N. doi:10.1139/p85-209.
- Lotz, H. T.; Schouten, J. A. (19 June 2001). "Phase behavior of the N2-Ar system at high pressures: A Raman spectroscopy study". Physical Review B. 64 (2): 024103. Bibcode:2001PhRvB..64b4103L. doi:10.1103/PhysRevB.64.024103.
- Kim, Minseob; Yoo, Choong-Shik (2011). "Highly repulsive interaction in novel inclusion D2–N2 compound at high pressure: Raman and x-ray evidence". The Journal of Chemical Physics. 134 (4): 044519. Bibcode:2011JChPh.134d4519K. doi:10.1063/1.3533957. PMID 21280760.
- Sihachakr, D.; Loubeyre, P. (15 October 2004). "O2 / N2 mixtures under pressure: A structural study of the binary phase diagram at 295 K". Physical Review B. 70 (13): 134105. Bibcode:2004PhRvB..70m4105S. doi:10.1103/PhysRevB.70.134105.
- Wu, Yu-Jong; Chen, Hui-Fen; Chuang, Shiang-Jiun; Huang, Tzu-Ping (10 December 2013). "Far Ultraviolet Absorption Spectra of N3 AND N2+ Generated by Electrons Impacting Gaseous N 2". The Astrophysical Journal. 779 (1): 40. Bibcode:2013ApJ...779...40W. doi:10.1088/0004-637X/779/1/40.
- Sansinena, M; Santos, MV; Zaritzky, N; Chirife, J (May 2012). "Comparison of heat transfer in liquid and slush nitrogen by numerical simulation of cooling rates for French straws used for sperm cryopreservation". Theriogenology. 77 (8): 1717–1721. doi:10.1016/j.theriogenology.2011.10.044. PMID 22225685.
- Schutte, Eliane; Picciolo, Grace Lee; Kaplan, David S. (2004). Tissue Engineered Medical Products (TEMPs). ASTM International. p. 8. ISBN 9780803134713.
- Porcu, Eleonora; Ciotti, Patrizia; Venturoli, Stefano (2012-12-06). Handbook of Human Oocyte Cryopreservation. Cambridge University Press. p. 33. ISBN 9781139851022.
- Becker, Edwin D.; Pimentel, George C. (1956). "Spectroscopic Studies of Reactive Molecules by the Matrix Isolation Method". The Journal of Chemical Physics. 25 (2): 224. Bibcode:1956JChPh..25..224B. doi:10.1063/1.1742860.
- Ozin, Geoffrey A.; Voet, Anthony Vander (15 October 1973). "Binary Dinitrogen Complexes of Rhodium, Rh(N2)n (where n= 1–4), in Low Temperature Matrices". Canadian Journal of Chemistry. 51 (20): 3332–3343. doi:10.1139/v73-498.
- "Neptune: Moons: Triton". NASA. Archived from the original on October 5, 2011. Retrieved September 21, 2007.
External links
 Media related to Solid nitrogen at Wikimedia Commons
Media related to Solid nitrogen at Wikimedia Commons- Jessica Orwig: Freezing Liquid Nitrogen Creates Something Amazing. On: BusinessInsider. Jan 28, 2015 - Videos of nitrogen boiling, freezing, and spontaneously changing crystal form.
- Xiaoli Wang, J. Li, N. Xu et al. (2015): Layered polymeric nitrogen in RbN3 at high pressures. In: Scientific Reports volume 5, Article number: 16677. doi:10.1038/srep16677.
