Periodic trends
Periodic trends are specific patterns in the properties of chemical elements that are revealed in the periodic table of elements. Major periodic trends include electronegativity, ionization energy, electron affinity, atomic radii, ionic radius, metallic character, and chemical reactivity.
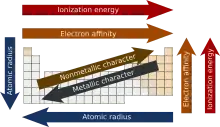
Periodic trends from the changes in the atomic structure of the chemical elements within their respective periods (horizontal rows) and groups in the periodic table. These laws enable the chemical elements to be organized in the periodic table based on their atomic structures and properties. Due to the periodic trends, the unknown properties of any element can be partially known.
Several exceptions, however, do exist, such as that of ionization energy in group 3, The electron affinity trend of group 17, the density trend of alkali metals aka group 1 elements and so on.
Periodic trends
The periodic trends are based on the Periodic Law, which states that if the chemical elements are listed in order of increasing atomic number, many of their properties go through cyclical changes, with elements of similar properties recurring at intervals.[1] For example, after arranging elements in their increasing atomic numbers, many of the physical and chemical properties of Lithium, such as its vigorous reactivity with water, recur in sodium, potassium and cesium.
This principle was discovered by Russian chemist Dmitri Mendeleev in 1871 after a number of investigations by scientists in the 19th century. Mendeleev also proposed a periodic system of elements that was based not only on atomic weights but also on the chemical and physical properties of the elements and their compounds.[2] In 1913, Henry Moseley determined that periodicity depends on the atomic number rather than atomic weight. Lothar Meyer presented his table several months after Mendeleev, but opposed his Periodic law. Initially, no theoretical explanation for the Periodic Law was available and it was used only as an empirical principle, but, with the development of quantum mechanics, it became possible to understand the theoretical basis for the Periodic Law.
The periodic recurrence of elements with similar physical and chemical properties, when the elements are listed in order of increasing atomic number, results directly from the periodic recurrence of similar electronic configurations in the outer shells of respective atoms.
The Discovery of Periodic Law constitutes one of the most important events in the history of chemical science. Almost every chemist makes extensive and continued use of Periodic Law. Periodic Law also led to the development of the periodic table, which is widely used nowadays.
Atomic radius
The atomic radius is the distance from the atomic nucleus to the outermost stable electron orbital in an atom that is at equilibrium. The atomic radius tends to decrease across a period from left to right due to the shrinking of the atom because of increasing effective nuclear force on the electrons. The atomic radius usually increases while going down a group due to the addition of a new energy level (shell which causes shrinkage in the size of the atoms across the period). However, atomic radii tend to increase diagonally, since the number of electrons has a larger effect than the sizeable nucleus. For example, lithium (145 picometer) has a smaller atomic radius than magnesium (150 picometer).
There are 4 types of atomic radius:-
- Covalent radius: half the distance between two atoms of a diatomic compound, singly bonded.
- Van der Waals radius: half the distance between the nuclei of atoms of different molecules in a lattice of covalent molecules.
- Metallic radius: half the distance between two adjacent nuclei of atoms in a metallic lattice.
- Ionic radius: half the distance between two nuclei of elements of an ionic compound.
Ionization energy
The ionization potential is the minimum amount of energy required to remove one electron from each atom in a mole of an isolated, neutral, and gaseous atom. The first ionization energy is the energy required to remove the first electron, and generally the nth ionization energy is the energy required to remove the atom's nth electron, after the (n−1) electrons before it has been removed. Trend-wise, ionization energy tends to increase while one progresses across a period because the greater number of protons (higher nuclear charge) attracts the orbiting electrons more strongly, thereby increasing the energy required to remove one of the electrons. Ionization energy and ionization potentials are completely different. The potential is an intensive property and it is measured by "volt"; whereas the energy is an extensive property expressed by "eV" or "kJ/mole".
As one progresses down a group on the periodic table, the ionization energy will likely decrease since the valence electrons are farther away from the nucleus and experience a weaker attraction to the nucleus's positive charge. There will be an increase of ionization energy from left to right of a given period and a decrease from top to bottom. As a rule, it requires far less energy to remove an outer-shell electron than an inner-shell electron. As a result, the ionization energies for a given element will increase steadily within a given shell, and when starting on the next shell down will show a drastic jump in ionization energy. Simply put, the lower the principal quantum number, the higher the ionization energy for the electrons within that shell. The exceptions are the elements in the boron and oxygen family, which require slightly less energy than the general trend.
Electron affinity
The electron affinity of an atom can be described either as the energy released by an atom when an electron is added to it, conversely as the energy required to detach an electron from a singly charged anion.[3] The sign of the electron affinity can be quite confusing, as atoms that become more stable with the addition of an electron (and so are considered to have a higher electron affinity) show a decrease in potential energy; i.e. the energy gained by the atom appears to be negative. In such a case, the atom’s electron affinity is positive. For atoms that become less stable upon gaining an electron, potential energy increases, which implies that the atom gains energy. In such a case, the atom's electron affinity is negative.[4] However, in the reverse scenario where electron affinity is defined as the energy required to detach an electron from an anion, the energy value obtained will be of the same magnitude but have the opposite sign. This is because those atoms with a high electron affinity are less inclined to give up an electron, and so take more energy to remove the electron from the atom. In this case, the atom with the more positive energy value has a higher electron affinity. As one progresses from left to right across a period, the electron affinity will increase.
Although it may seem that fluorine should have the greatest electron affinity, the small size of fluorine generates enough repulsion that chlorine (Cl) has the greatest electron affinity.
Electronegativity
Electronegativity is a measure of the ability of an atom or molecule to attract pairs of electrons in the context of a chemical bond.[5] The type of bond formed is largely determined by the difference in electronegativity between the atoms involved, using the Pauling scale. Trend-wise, as one moves from left to right across a period in the periodic table, the electronegativity increases due to the stronger attraction that the atoms obtain as the nuclear charge increases. Moving down in a group, the electronegativity decreases due to an increase in the distance between the nucleus and the valence electron shell, thereby decreasing the attraction, making the atom have less of an attraction for electrons or protons.
However, in the group (iii) elements electronegativity increases from aluminium to thallium.
Valence electrons
Valence electrons are the electrons in the outermost electron shell of an isolated atom of an element. Sometimes, it is also regarded as the basis of Modern Periodic Table. In a period, the number of valence electrons increases (mostly for light metal/elements) as we move from left to right side. However, in a group this periodic trend is constant, that is the number of valence electrons remains the same.
Valency
Valency in the periodic table across a period first increases and then decreases. There is no change going down a group.
However, this periodic trend is sparsely followed for heavier elements (elements with atomic number greater than 20), especially for lanthanide and actinide series.
The greater the number of core electrons, the greater the shielding of electrons from the core charge of the nucleus. For this reason ionization energy is lower for elements lower down in a group, and polarizability of species is higher for elements lower down in a group. The valency does not change going down a group since the bonding behavior is not affected by the core electrons. However, non-bonding interactions such as those just cited are affected by core electrons.
Metallic and non-metallic properties
Metallic properties increase down groups as decreasing attraction between the nuclei and the outermost electrons cause the outermost electrons to be loosely bound and thus able to conduct heat and electricity. Across the period, from left to right, the increasing attraction between the nuclei and the outermost electrons causes the metallic character to decrease.
Non-metallic property increases across a period and decreases down the group due to the same reason due to an increase in nuclear attractive force. Metals are ductile while nonmetals are not.
Further reading
References
- Harry H. Sister (1963). Electronic structure, properties, and the periodic law. New York: Reinhold publishing corporation.
The physical and chemical properties of elements are periodic functions of the charges on their atomic nuclei i.e. their atomic numbers.
- Sauders, Nigel (2015). Who Invented The Periodic Table?. Encyclopedia Britannica. pp. 26–29. ISBN 9781625133168.
- Rennie, Richard; Law, Jonathan (2019). A Dictionary of Physics. Oxford University Press. ISBN 9780198821472.
- SparkNotes Editors (27 November 2015). "SparkNote on Atomic Structure". SparkNotes.com. Retrieved 29 November 2015.
- Allred, A. Louis (2014). Electronegativity. McGraw-Hill Education. ISBN 9780071422895.