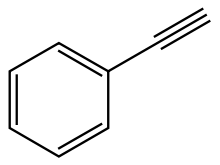
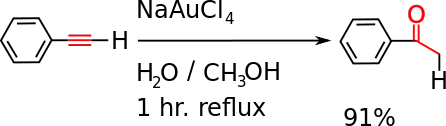Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ethynylbenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.861 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6 | |
| Molar mass | 102.133 g/mol |
| Density | 0.93 g/cm3 |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
| Acidity (pKa) | 28.7 (DMSO),[1] 23.2 (aq, extrapolated)[2] |
| -72.01·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
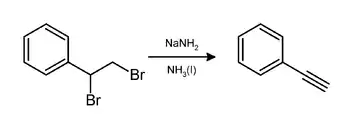
In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia:[3]
It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide.[4]
Reactions
- Phenylacetylene can be reduced (hydrogenated) by hydrogen over Lindlar catalyst to give styrene.
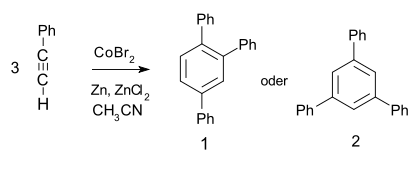
- It undergoes a metal catalyzed trimerization to give 1,2,4- (97%) and 1,3,5-triphenylbenzene:[5]
- Choosing a rhodium catalyst leads to the cyclotrimerization producing both the 1,2,4- and 1,3,5- isomers along with substantial quantities of acyclic enyne dimer products:[6]
- It undergoes a hydration reaction, catalyzed by gold(III) or mercury(II), to give acetophenone.
See also
References
- Bordwell, F.G. Acc. Chem. Res. 1988, 21, 456-463.
- Streitwieser, A.,Jr.; Ruben, D.M.E; J. Am. Chem. Soc. 1971., 93, 1794-1795.
- Kenneth N. Campbell, Barbara K. Campbell (1950). "Phenylacetylene". Organic Syntheses. 30: 72. doi:10.15227/orgsyn.030.0072.
- John C. Hessler (1922). "Phenylacetylene". Organic Syntheses. 2: 67. doi:10.15227/orgsyn.002.0067.
- Gerhard Hilt; Thomas Vogler; Wilfried Hess; Fabrizio Galbiati (2005). "A simple cobalt catalyst system for the efficient and regioselective cyclotrimerisation of alkynes". Chemical Communications. 2005 (11): 1474–1475. doi:10.1039/b417832g. PMID 15756340.
- Ardizzoia, G. A.; Brenna, S.; Cenini, S.; LaMonica, G.; Masciocchi, N.; Maspero, A. (2003). "Oligomerization and Polymerization of Alkynes Catalyzed by Rhodium(I) Pyrazolate Complexes". Journal of Molecular Catalysis A: Chemical. 204–205: 333–340. doi:10.1016/S1381-1169(03)00315-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.