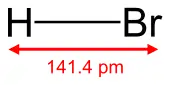
Hydrogen bromide
Hydrogen bromide is the heteronuclear diatomic molecular compound with the formula HBr, a hydrogen halide consisting of hydrogen and bromine. In pure form it is a colorless gas.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hydrogen bromide | |||
| Systematic IUPAC name
Bromane[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3587158 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.090 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Hydrobromic+Acid | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1048 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| HBr | |||
| Molar mass | 80.91 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Acrid | ||
| Density | 3.6452 kg/m3 (0 °C, 1013 mbar)[2] | ||
| Melting point | −86.9 °C (−124.4 °F; 186.2 K) | ||
| Boiling point | −66.8 °C (−88.2 °F; 206.3 K) | ||
| 221 g/100 mL (0 °C) 204 g/100 mL (15 °C) 193 g/100 mL (20 °C) 130 g/100 mL (100 °C) | |||
| Solubility | Soluble in alcohol, organic solvents | ||
| Vapor pressure | 2.308 MPa (at 21 °C) | ||
| Acidity (pKa) | −8.8 (±0.8);[3] ~−9[4] | ||
| Basicity (pKb) | ~23 | ||
| Conjugate acid | Bromonium | ||
| Conjugate base | Bromide | ||
Refractive index (nD) |
1.325 | ||
| Structure | |||
| Linear | |||
| 820 mD | |||
| Thermochemistry | |||
Heat capacity (C) |
350.7 mJ/(K·g) | ||
Std molar entropy (S |
198.696–198.704 J/(K·mol)[5] | ||
Std enthalpy of formation (ΔfH⦵298) |
−36.45...−36.13 kJ/mol[5] | ||
| Hazards | |||
| Safety data sheet | hazard.com | ||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
| H314, H335 | |||
| P261, P280, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
2858 ppm (rat, 1 h) 814 ppm (mouse, 1 h)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 3 ppm (10 mg/m3)[7] | ||
REL (Recommended) |
TWA 3 ppm (10 mg/m3)[7] | ||
IDLH (Immediate danger) |
30 ppm[7] | ||
| Related compounds | |||
Related compounds |
Hydrogen fluoride Hydrogen chloride Hydrogen iodide Hydrogen astatide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydrogen bromide is very soluble in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
Both the anhydrous and aqueous solutions of HBr are common reagents in the preparation of bromide compounds.
Uses of HBr
Hydrogen bromide and hydrobromic acid are important reagents in the production of inorganic and organic bromine compounds.[8] The free-radical addition of HBr to alkenes gives alkyl bromides:
- RCH=CH2 + HBr → R−CHBr−CH3
These alkylating agents are precursors to fatty amine derivatives. Similar free radical addition to allyl chloride and styrene gives 1-bromo-3-chloropropane and phenylethylbromide, respectively.
Hydrogen bromide reacts with dichloromethane to give bromochloromethane and dibromomethane, sequentially:
- HBr + CH2Cl2 → HCl + CH2BrCl
- HBr + CH2BrCl → HCl + CH2Br2
Allyl bromide is prepared by treating allyl alcohol with HBr:
- CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O
Other reactions
Although not widely used industrially, HBr adds to alkenes to give bromoalkanes, an important family of organobromine compounds. Similarly, HBr adds to haloalkene to form a geminal dihaloalkane. (This type of addition follows Markovnikov's rule):
- RC(Br)=CH2 + HBr → RC(Br2)−CH3
HBr also adds to alkynes to yield bromoalkenes. The stereochemistry of this type of addition is usually anti:
- RC≡CH + HBr → RC(Br)=CH2
Also, HBr is used to open epoxides and lactones and in the synthesis of bromoacetals. Additionally, HBr catalyzes many organic reactions.[9][10][11][12]
Potential applications
HBr has been proposed for use in a utility-scale flow-type battery.[13]
Industrial preparation
Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °C. The reaction is typically catalyzed by platinum or asbestos.[10][14]
Laboratory synthesis
HBr can be synthesized by a variety of methods. It may be prepared in the laboratory by distillation of a solution of sodium bromide or potassium bromide with phosphoric acid or sulfuric acid:[15]
- KBr + H2SO4 → KHSO4 + HBr
Concentrated sulfuric acid is less effective because it oxidizes HBr to bromine:
- 2 HBr + H2SO4 → Br2 + SO2 + 2 H2O
The acid may be prepared by:
- reaction of bromine with water and sulfur:[15]
- 2 Br2 + S + 2 H2O → 4 HBr + SO2
- bromination of tetralin:[15]
- C10H12 + 4 Br2 → C10H8Br4 + 4 HBr
- reduction of bromine with phosphorous acid:[10]
- Br2 + H3PO3 + H2O → H3PO4 + 2 HBr
Anhydrous hydrogen bromide can also be produced on a small scale by thermolysis of triphenylphosphonium bromide in refluxing xylene.[9]
Hydrogen bromide prepared by the above methods can be contaminated with Br2, which can be removed by passing the gas through a solution of phenol at room temperature in tetrachloromethane or other suitable solvent (producing 2,4,6-tribromophenol and generating more HBr in the process) or through copper turnings or copper gauze at high temperature.[14]
Safety
HBr is highly corrosive and irritating to inhalation.
References
- "Hydrobromic Acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 10 November 2011.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Trummal, Aleksander; Lipping, Lauri; Kaljurand, Ivari; Koppel, Ilmar A; Leito, Ivo (2016). "Acidity of Strong Acids in Water and Dimethyl Sulfoxide". The Journal of Physical Chemistry A. 120 (20): 3663–9. Bibcode:2016JPCA..120.3663T. doi:10.1021/acs.jpca.6b02253. PMID 27115918.
- Perrin, D. D. Dissociation constants of inorganic acids and bases in aqueous solution. Butterworths, London, 1969.
- Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 978-0-618-94690-7.
- "Hydrogen bromide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0331". National Institute for Occupational Safety and Health (NIOSH).
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.CS1 maint: multiple names: authors list (link)
- Hercouet, A.; LeCorre, M. (1988) Triphenylphosphonium bromide: A convenient and quantitative source of gaseous hydrogen bromide. Synthesis, 157–158.
- Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heineman: Oxford, Great Britain; 1997; pp. 809–812.
- Carlin, William W. U.S. Patent 4,147,601, April 3, 1979.
- Vollhardt, K. P. C.; Schore, N. E. Organic Chemistry: Structure and Function; 4th Ed.; W. H. Freeman and Company: New York, NY; 2003.
- https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/30535ag.pdf
- Ruhoff, J. R.; Burnett, R. E.; Reid, E. E. "Hydrogen Bromide (Anhydrous)" Organic Syntheses, Vol. 15, p. 35 (Coll. Vol. 2, p. 338).
- M. Schmeisser "Chlorine, Bromine, Iodine" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 282.