Pinoline
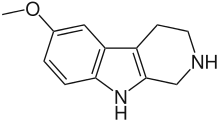
Pinoline is a methoxylated tryptoline (5-methoxytryptoline) long claimed to be produced in the pineal gland during the metabolism of melatonin, however its pineal occurrence remains controversial.[1] Its IUPAC name is 6-methoxy-1,2,3,4-tetrahydro-β-carboline, usually abbreviated as 6-MeO-THBC, and its more common name is a combination of "pineal beta-carboline".[2] The biological activity of this molecule is of interest as a potential free radical scavenger, also known as an antioxidant,[3] and as a monoamine oxidase A inhibitor.[4]
 | |
| Names | |
|---|---|
| IUPAC name
6-Methoxy-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole | |
| Other names
6-MeO-THBC; 5-MeO-TLN; 6-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole; Pinoline; 6-Methoxy-2,3,4,9-tetrahydro-1H-β-carboline; 6-Methoxy-1,2,3,4-tetrahydro-β-carboline; 6-Methoxy-tetrahydronorharman; 6-Methoxy-2,3,4,9-tetrahydro-1H-β-carboline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.161.873 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H14N2O | |
| Molar mass | 202.257 g·mol−1 |
| Melting point | 216 to 224 °C (421 to 435 °F; 489 to 497 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bausch & Lomb filed a patent for a drug delivery device utilizing this molecule, designed to treat various ophthalmic disorders in 2006.[5]
Pharmacology
One of pinoline's pharmacological properties is its ability to promote neurogenesis in vitro; even at trace concentrations.[6]
Aluminium toxicity causes an increase in lipid peroxidation, with most damage occurring in the brain. A recent review of studies shows pinoline and melatonin to be effective at reducing the lipid peroxidation. Studies included both human and animal subjects. The studies’ results support that pinoline has antioxidant properties.
Lipopolysaccharide is produced by Gram-negative bacteria and stimulates the production of free radicals which in turn cause lipid peroxidation. A recent study compared the effectiveness of melatonin and other similar compounds on the lipopolysaccharide induced lipid peroxidation. The results showed support for pinoline’s ability to reduce damage from lipid peroxidation. Pinoline was also shown to be more effective than vitamin E at reducing lipopolysaccharide activity in the retina.[7]
Another recent study compared the antioxidant properties of compounds from the tryptophan metabolic pathway in the pineal gland against oxidative damage to the lipids and proteins of synaptosomes. Synaptosomes isolated from rat brains were used in an experiment assessing damage by measuring malondialdehyde, 4-hydroxyalkenal, and carbonyl content in the proteins. Pinoline was shown to be the most powerful antioxidant. These results support the evidence for pinoline’s antioxidant abilities and the potential to protect against oxidative damage.[8]
See also
References
- Barker, Steven A. (2013). "LC/MS/MS analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate" (PDF). Biomedical Chromatography. 27 (12): 1690–1700. doi:10.1002/bmc.2981. hdl:2027.42/101767. PMID 23881860.
- Callaway, James C.; Gyntber, Jukka; Poso, Antti; Airaksinen, Mauno M.; Vepsäläinen, Jouko (1994). "The pictet-spengler reaction and biogenic tryptamines: Formation of tetrahydro-β-carbolines at physiologicalpH". Journal of Heterocyclic Chemistry. 31 (2): 431. doi:10.1002/jhet.5570310231.
- Schiller, Erich; Bartsch, H. (2003). Free Radicals and Inhalation Pathology: Respiratory System, Mononuclear Phagocyte System, Hypoxia and Reoxygenation, Pneumoconioses, and Other Granulomatoses, Cancer (Google Books, page view). Springer. p. 107. ISBN 978-3-540-00201-7. Retrieved 2009-02-14.
- Airaksinen, M. M., Huang, J. T., Ho, B. T., Taylor, D., and Walker, K. (1978). "The Uptake of 6-Methoxy-1,2,3,4-Tetrahydro-β-carboline and its Effect on 5-Hydroxytryptamine Uptake and Release in Blood Platelets". Acta Pharmacologica et Toxicologica. 43 (5): 375–380. doi:10.1111/j.1600-0773.1978.tb02281.x. PMID 726902.CS1 maint: multiple names: authors list (link)
- Bartels, S. P. (2006) U.S. Patent No. 20,060,292,202 Washington, DC: U.S.
- { Mario de la Fuente et al. 2015 "Neurogenic Potential Assessment and Pharmacological Characterization of 6-Methoxy-1,2,3,4-tetrahydro-β-carboline (Pinoline) and Melatonin–Pinoline Hybrids" http://pubs.acs.org/doi/abs/10.1021/acschemneuro.5b00041}
- Sewerynek, E; Wiktorska, JA; Stuss, M (2011). "6-methoxytryptophol reduces lipopolysaccharide-induced lipid peroxidation in vitro more effectively than melatonin". Journal of Physiology and Pharmacology. 62 (6): 677–83. PMID 22314571.
- Millán-Plano, Sergio; Piedrafita, Eduardo; Miana-Mena, Francisco J.; Fuentes-Broto, Lorena; Martínez-Ballarín, Enrique; López-Pingarrón, Laura; Sáenz, María A.; García, Joaquín J. (2010). "Melatonin and Structurally-Related Compounds Protect Synaptosomal Membranes from Free Radical Damage". International Journal of Molecular Sciences. 11 (1): 312–28. doi:10.3390/ijms11010312. PMC 2821006. PMID 20162018.
