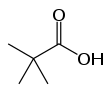
Pivalic acid
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. A common abbreviation for the pivalyl or pivaloyl group (t-BuC(O)) is Piv and for pivalic acid (t-BuC(O)OH) is PivOH.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethylpropanoic acid | |||
| Other names
Pivalic acid Dimethylpropanoic acid Neopentanoic acid Trimethylacetic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.839 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H10O2 | |||
| Molar mass | 102.133 g·mol−1 | ||
| Density | 0.905 g/cm3 | ||
| Melting point | 35 °C (95 °F; 308 K) | ||
| Boiling point | 163.7 °C (326.7 °F; 436.8 K) | ||
| Related compounds | |||
Related compounds |
neopentyl alcohol neopentane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Industrial route
Pivalic acid is prepared by hydrocarboxylation of isobutene via the Koch reaction:
- (CH3)2C=CH2 + CO + H2O → (CH3)3CCO2H
Such reactions require an acid catalyst such as hydrogen fluoride. tert-Butyl alcohol and isobutyl alcohol can also be used in place of isobutene. Globally, several million kilograms are produced annually.[1] Pivalic acid is also economically recovered as a by-product from the production of semi-synthetic penicillins like ampicillin and amoxycillin.
Laboratory methods
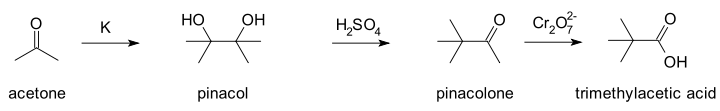
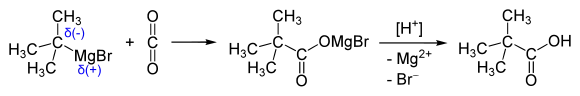
It was originally prepared by the oxidation of pinacolone with chromic acid[2] and by the hydrolysis of tert-butyl cyanide.[3] Convenient laboratory routes proceed via tert-butyl chloride via carbonation of the Grignard reagent[4] and by oxidation of pinacolone.[5]
Applications
Relative to esters of most carboxylic acids, esters of pivalic acid are unusually resistant to hydrolysis. Some applications result from this thermal stability. Polymers derived from pivalate esters of vinyl alcohol are highly reflective lacquers. The pivaloyl (abbreviated Piv or Pv) group is a protective group for alcohols in organic synthesis. Pivalic acid is sometimes used as an internal chemical shift standard for NMR spectra of aqueous solutions. While DSS is more commonly used for this purpose, the minor peaks from protons on the three methylene bridges in DSS can be problematic. The 1H NMR spectrum at 25 °C and neutral pH is a singlet at 1.08 ppm. Pivalic acid is employed as co-catalyst in some of the Palladium catalyzed C-H functionalization reaction.[6][7]
Alcohol protection
The pivaloyl group is used as a protecting group in organic synthesis. Common protection methods include treatment of alcohol with pivaloyl chloride (PvCl) in presence of pyridine.[8]
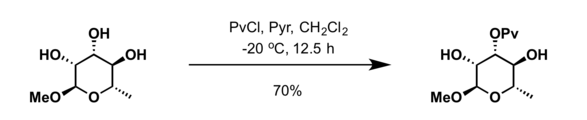
Alternatively, the esters can be prepared using pivaloic anhydride in the presence of scandium triflate (Sc(OTf)3) or vanadyl triflate (VO(OTf)2).
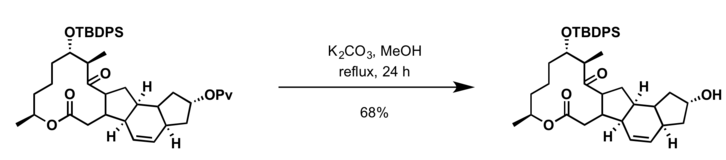
Common deprotection methods involve hydrolysis with a base or other nucleophiles.[9][10][11][12]
See also
References
- Riemenschneider, Wilhelm (2000). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_235. ISBN 3527306730.
- Friedel and Silva, Ber. 6, 146, 826 (1873).
- Butlerow, Ann. 165, 322 (1873).
- S. V. Puntambeker; E. A. Zoellner; L. T. Sandborn; E. W. Bousquet (1941). "Trimethylacetic acid from tert.- Butyl Chloride". Organic Syntheses. doi:10.15227/orgsyn.008.0104.; Collective Volume, 1, p. 524
- L. T. Sandborn; E. W. Bousquet (1941). "Trimethylacetic acid from Pinacolone". Organic Syntheses. doi:10.15227/orgsyn.008.0104.; Collective Volume, 1, p. 524
- Lafrance, Marc; Fagnou, Keith (2006-12-27). "Palladium-catalyzed benzene arylation: incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design". Journal of the American Chemical Society. 128 (51): 16496–16497. doi:10.1021/ja067144j. ISSN 0002-7863. PMID 17177387.
- Zhao, Dongbing; Wang, Weida; Lian, Shuang; Yang, Fei; Lan, Jingbo; You, Jingsong (2009-01-26). "Phosphine-Free, Palladium-Catalyzed Arylation of Heterocycles through CH Bond Activation with Pivalic Acid as a Cocatalyst". Chemistry – A European Journal. 15 (6): 1337–1340. doi:10.1002/chem.200802001. ISSN 0947-6539.
- Robins, Morris J.; Hawrelak, S. D.; Kanai, Tadashi; Siefert, Jan Marcus; Mengel, Rudolf (1979). "Nucleic acid related compounds. 30. Transformations of adenosine to the first 2',3'-aziridine-fused nucleosides, 9-(2,3-epimino-2,3-dideoxy-.beta.-D-ribofuranosyl)adenine and 9-(2,3-epimino-2,3-dideoxy-.beta.-D-lyxofuranosyl)adenine". The Journal of Organic Chemistry. 44 (8): 1317–22. doi:10.1021/jo01322a026.
- Van Boeckel, C.A.A.; Van Boom, J.H. (1979). "Synthesis of glucosylphosphatidylglycerol via a phosphotriester intermediate". Tetrahedron Letters. 20 (37): 3561–4. doi:10.1016/S0040-4039(01)95462-0.
- Griffin, B.E.; Jarman, M.; Reese, C.B. (1968). "The Synthesis of oligoribonucleotides—IV". Tetrahedron. 24 (2): 639–62. doi:10.1016/0040-4020(68)88015-9. PMID 5637486.
- Ogilvie, Kelvin K.; Iwacha, Donald J. (1973). "Use of the tert-butyldimethylsilyl group for protecting the hydroxyl functions of nucleosides". Tetrahedron Letters. 14 (4): 317–9. doi:10.1016/S0040-4039(01)95650-3.
- Paquette, Leo A.; Collado, Iván; Purdie, Mark (1998). "Total Synthesis of Spinosyn A. 2. Degradation Studies Involving the Pure Factor and Its Complete Reconstitution". Journal of the American Chemical Society. 120 (11): 2553–62. doi:10.1021/ja974010k. INIST:10388970.