Vinyl alcohol
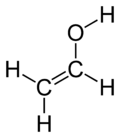
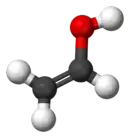
Vinyl alcohol, also called ethenol (IUPAC name), is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde. It is not a precursor to polyvinyl alcohol.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethenol | |||
| Other names
Hydroxyethene Hydroxyethylene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.350 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4O | |||
| Molar mass | 44.053 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
Vinyl alcohol can be formed by the pyrolytic elimination of water from ethylene glycol at a temperature of 900 °C and low pressure.[1]
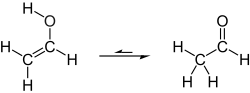
Tautomerization of vinyl alcohol to acetaldehyde
Under normal conditions, vinyl alcohol converts (tautomerizes) to acetaldehyde:
At room temperature, acetaldehyde (H3CC(O)H) is more stable than vinyl alcohol (H2C=CHOH) by 42.7 kJ/mol:[2]
- H2C=CHOH → H3CC(O)H ΔH298,g = −42.7 kJ/mol
The uncatalyzed keto–enol tautomerism by a 1,3-hydrogen migration is forbidden by the Woodward–Hoffmann rules and therefore has a high activation barrier and is not a significant pathway at or near room temperature. However, even trace amounts of acids or bases (including water) can catalyze the reaction. Even with rigorous precautions to minimize adventitious moisture or proton sources, vinyl alcohol can only be stored for minutes to hours before it isomerizes to acetaldehyde. (Carbonic acid is another example of a substance that is kinetically stable when rigorously pure, but decomposes rapidly due to catalysis by trace moisture.)
The tautomerization can also be catalyzed via photochemical process. These findings suggest that the keto–enol tautomerization is a viable route under atmospheric and stratospheric conditions, relevant to a role for vinyl alcohol in the production of organic acids in the atmosphere.[4][5]
Vinyl alcohol can be stabilized by controlling the water concentration in the system and utilizing the kinetic favorability of the deuterium-produced kinetic isotope effect (kH+/kD+ = 4.75, kH2O/kD2O = 12). Deuterium stabilization can be accomplished through hydrolysis of a ketene precursor in the presence of a slight stoichiometric excess of heavy water (D2O). Studies show that the tautomerization process is significantly inhibited at ambient temperatures ( kt ≈ 10−6 M/s), and the half-life of the enol form can easily be increased to t1/2 = 42 minutes for first-order hydrolysis kinetics.[6]
Relationship to poly(vinyl alcohol)
Because of the instability of vinyl alcohol, the thermoplastic polyvinyl alcohol (PVA or PVOH) is made indirectly by polymerization of vinyl acetate followed by hydrolysis of the ester bonds (Ac = acetyl; HOAc = acetic acid):
- n CH2=CHOAc → (CH2−CHOAc)n
- (CH2−CHOAc)n + n H2O → (CH2−CHOH)n + n HOAc
As a ligand
Several metal complexes are known that contain vinyl alcohol as a ligand. One example is Pt(acac)(η2-C2H3OH)Cl.[7]
Occurrence in interstellar medium
Vinyl alcohol was detected in the molecular cloud Sagittarius B.[8] Its stability in the (dilute) interstellar medium shows that its tautomerization does not happen unimolecularly.
References
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart. Organic Chemistry, 2nd edition, pp. 456-57. Oxford University Press, 2012. ISBN 978-0-19-927029-3.
- R.D. Johnson III. "CCCBDB NIST Standard Reference Database". Retrieved 2014-08-30.
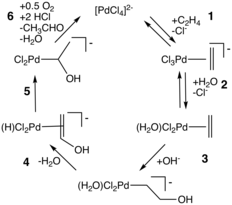
- J. A. Keith, P. M. Henry (2009). "The Mechanism of the Wacker Reaction: A Tale of Two Hydroxypalladations". Angew. Chem. Int. Ed. 48: 9038–9049. doi:10.1002/anie.200902194. PMID 19834921.CS1 maint: uses authors parameter (link)
- Heazlewood, B. R.; Maccarone, A. T.; Andrews, D. U.; Osborn, D. L.; Harding, L. B.; Klippenstein, S. J.; Jordan, M. J. T.; Kable, S. H. "Near-threshold H/D exchange in CD3CHO photodissociation." Nat. Chem. 2011, 3, 443−448. doi:10.1038/nchem.1052
- Andrews D. U., Heazlewood B. R., Maccarone A. T., Conroy T., Payne R. J., Jordan M. J. T., Kable S. H. (2012). "Photo-tautomerization of acetaldehyde to vinyl alcohol: A potential route to tropospheric acids". Science. 337: 1203–1206. doi:10.1126/science.1220712. PMID 22903524.CS1 maint: multiple names: authors list (link)
- Cederstav, Anna K.; Novak, Bruce M. (1994). "Investigations into the Chemistry of Thermodynamically Unstable Species. The Direct Polymerization of Vinyl Alcohol, the Enolic Tautomer of Acetaldehyde". Journal of the American Chemical Society. 100 (9): 4073–4074. doi:10.1021/ja00088a051.
- Cotton F. A., Francis J. N., Frenz B. A., Tsutsui M. (1973). "Structure of a dihapto(vinyl alcohol) complex of platinum(II)". Journal of the American Chemical Society. 95: 2483–6. doi:10.1021/ja00789a011.CS1 maint: multiple names: authors list (link)
- "Scientists Toast the Discovery of Vinyl Alcohol in Interstellar Space". National Radio Astronomy Observatory. 2001-10-01. Retrieved 2006-12-20.