Ponesimod
Ponesimod (INN, codenamed ACT-128800) is an experimental drug for the treatment of multiple sclerosis (MS) and psoriasis. It is being developed by Actelion.
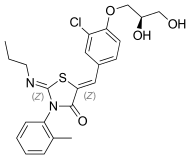 | |
 | |
| Clinical data | |
|---|---|
| Other names | ACT-128800 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | 2 main metabolites |
| Elimination half-life | 31–34 hrs[1] |
| Excretion | Feces (57–80%, 26% unchanged), urine (10–18%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H25ClN2O4S |
| Molar mass | 460.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clinical trials
In a 2009–2011 Phase II clinical trial including 464 MS patients, ponesimod treatment resulted in fewer new active brain lesions than placebo, measured during the course of 24 weeks.[3][4]
In a 2010–2012 Phase II clinical trial including 326 patients with psoriasis, 46 or 48% of patients (depending on dosage) had a reduction of at least 75% Psoriasis Area and Severity Index (PASI) score compared to placebo in 16 weeks.[3][5] The approval is already applied for in 2020.[6]
Adverse effects
Common adverse effects in studies were temporary bradycardia (slow heartbeat), usually at the beginning of the treatment, dyspnoea (breathing difficulties), and increased liver enzymes (without symptoms). No significant increase of infections was observed under ponesimod therapy.[3] QT prolongation is detectable but was considered to be too low to be of clinical importance in a study.[7]
Mechanism of action
Like fingolimod, which is already approved for the treatment of MS, ponesimod blocks the sphingosine-1-phosphate receptor. This mechanism prevents lymphocytes (a type of white blood cells) from leaving lymph nodes.[3] Ponesimod is selective for subtype 1 of this receptor, S1P1.[8]
See also
References
- Brossard P, Scherz M, Halabi A, Maatouk H, Krause A, Dingemanse J (February 2014). "Multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ponesimod, an S1P1 receptor modulator: favorable impact of dose up-titration". Journal of Clinical Pharmacology. 54 (2): 179–88. doi:10.1002/jcph.244. PMID 24408162.
- Reyes M, Hoch M, Brossard P, Wagner-Redeker W, Miraval T, Dingemanse J (February 2015). "Mass balance, pharmacokinetics and metabolism of the selective S1P1 receptor modulator ponesimod in humans". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 45 (2): 139–49. doi:10.3109/00498254.2014.955832. PMID 25188442. S2CID 23905158.
- Spreitzer H (29 September 2014). "Neue Wirkstoffe – Ponesimod". Österreichische Apothekerzeitung (in German) (20/2014): 42.
- Olsson T, Boster A, Fernández Ó, Freedman MS, Pozzilli C, Bach D, et al. (November 2014). "Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial". Journal of Neurology, Neurosurgery, and Psychiatry. 85 (11): 1198–208. doi:10.1136/jnnp-2013-307282. PMC 4215282. PMID 24659797.
- Vaclavkova A, Chimenti S, Arenberger P, Holló P, Sator PG, Burcklen M, et al. (December 2014). "Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial". Lancet. 384 (9959): 2036–45. doi:10.1016/S0140-6736(14)60803-5. PMID 25127208. S2CID 20452934.
- DMSG, AMSEL e V.-Landesverband der (2020-07-06). "Ozanimod bei schubförmiger MS zugelassen". Multiple Sklerose News - AMSEL (in German). Retrieved 2020-10-03.
- Hoch M, Darpo B, Brossard P, Zhou M, Stoltz R, Dingemanse J (May 2015). "Effect of ponesimod, a selective S1P1 receptor modulator, on the QT interval in healthy individuals". Basic & Clinical Pharmacology & Toxicology. 116 (5): 429–37. doi:10.1111/bcpt.12336. PMID 25287214.
- "Ponesimod". Actelion. Archived from the original on 3 December 2011. Retrieved 31 October 2014.
