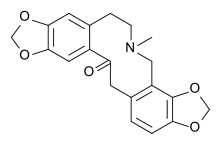
Protopine
Protopine is an alkaloid occurring in opium poppy,[2] Corydalis tubers[3] and other plants of the family papaveraceae, like Fumaria officinalis.[4] Protopine is metabolically derived from the benzylisoquinoline alkaloid (S)-Reticuline through a progressive series of five enzymatic transformations: 1) berberine bridge enzyme to (S)-Scoulerine; 2) (S)-cheilanthifoline synthase/CYP719A25 to (S)-Cheilanthifoline; 3) (S)-stylopine synthase/CYP719A20 to (S)-Stylopine; 4) (S)-tetrahydroprotoberberine N-methyltransferase to (S)-cis-N-Methylstylopine; and ultimately, 5) N-methylstylopine hydroxylase to protopine.[5]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
7-Methyl-6,8,9,16-tetrahydrobis[1,3]benzodioxolo[4,5-c:5′,6′-g]azecin-15(7H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.546 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H19NO5 | |
| Molar mass | 353.369 g/mol |
| Appearance | white crystals |
| Density | 1.399 g/cm3 |
| Melting point | 208 °C (406 °F; 481 K) |
| practically insoluble | |
| Solubility in chloroform | 1:15 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It has been found to inhibit histamine H1 receptors and platelet aggregation, and acts as an analgesic.[6][7]
See also
- Protopine 6-monooxygenase
- Cryptopine
- Bürgi-Dunitz angle
References
- The Merck Index (9 ed.). New Jersey: Merck & Co. 1976. p. 1023.
- The Free Dictionary: Protopine
- Jiang, B; Cao, K; Wang, R (2004). "Inhibitory effect of protopine on K(ATP) channel subunits expressed in HEK-293 cells". European Journal of Pharmacology. 506 (2): 93–100. doi:10.1016/j.ejphar.2004.11.004. PMID 15588728.
- Vrba, J.; Vrublova, E.; Modriansky, M.; Ulrichova, J. (2011). "Protopine and allocryptopine increase mRNA levels of cytochromes P450 1A in human hepatocytes and HepG2 cells independently of AhR". Toxicology Letters. 203 (2): 135–141. doi:10.1016/j.toxlet.2011.03.015. PMID 21419197.
- Hagel, Jillian M; Morris, Jeremy S; Lee, Eun-Jeong; Desgagne-Penix, Isabel; Bross, Crystal D; Chang, Limei; Chen, Xue; Farrow, Scott C; Zhang, Ye (2015). "Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants". BMC Plant Biology. 15: 227. doi:10.1186/s12870-015-0596-0. PMID 26384972.
- Saeed, SA; Gilani, AH; Majoo, RU; Shah, BH (1997). "Anti-thrombotic and anti-inflammatory activities of protopine". Pharmacological Research. 36 (1): 1–7. doi:10.1006/phrs.1997.0195. PMID 9368908.
- Protopine at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
