Riccardin C
Riccardin C is a macrocyclic bis(bibenzyl). It is a secondary metabolite isolated from the Siberian cowslip subspecies Primula veris subsp. macrocalyx,[1][2] in Reboulia hemisphaerica[3] and in the Chinese liverwort Plagiochasma intermedium.[4]
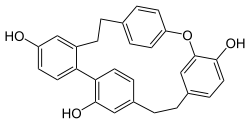 Riccardin C, flat molecule representation | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C28H24O4 | |
| Molar mass | 424.49 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In 2005, the compound was prepared by total synthesis together with the strained compound cavicularin.[5][6]
References
- Riccardin C, a bisbibenzyl compound from Primula macrocalyx. Yu. S. Kosenkova, M. P. Polovinka, N. I. Komarova, D. V. Korchagina, N. Yu. Kurochkina, V. A. Cheremushkina and N. F. Salakhutdinov, Chemistry Of Natural Compounds, Volume 43, Number 6, pages 712-713, doi:10.1007/s10600-007-0241-8
- Seasonal Dynamics of Riccardin C Accumulation in Primula macrocalyx Bge. Yu. S. Kosenkova, M.P. Polovinka, N.I. Komarova, D.V. Korchagina, N. Yu. Kurochkina, V.A. Cheremushkina and N.F. Salakhutdinov, Chemistry for Sustainable Development, 2009, 17, pages 507-511 (article)
- RiccardinC, a novel cyclic bibenzyl derivative from Reboulia hemisphaerica. Yoshinori Asakawa, Reiko Matsuda, Phytochemistry, Volume 21, Issue 8, 1982, Pages 2143–2144, doi:10.1016/0031-9422(82)83073-2
- Antifungal macrocyclic bis(bibenzyls) from the Chinese liverwort Ptagiochasm intermedlum L. Chun-Feng Xie, Jian-Bo Qu, Xiu-Zhen Wu, Na Liu, Mei Ji and Hong-Xiang Lou, Natural Product Research: Formerly Natural Product Letters, 2010, Volume 24, Issue 6, pages 515-520, doi:10.1080/14786410802271587
- David C. Harrowven; Timothy Woodcock; Peter D. Howes (2005). "Total Synthesis of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene That Adopts a Boat Configuration". Angewandte Chemie. 44 (25): 3899–3901. doi:10.1002/anie.200500466. PMID 15900530. Archived from the original on 2012-12-10.
- Kostiuk, S. L., Woodcock, T., Dudin, L. F., Howes, P. D. and Harrowven, D. C. (2011), Unified Syntheses of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene Adopting a Boat Configuration. Chemistry - A European Journal, 17: 10906–10915. doi:10.1002/chem.201101550
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.