Sexual mimicry
Sexual mimicry occurs when one sex mimics the opposite sex in its behavior, appearance, or chemical signalling. It is more commonly seen within invertebrate species, although sexual mimicry is also seen among vertebrates such as spotted hyenas. Sexual mimicry is commonly used as a mating strategy to gain access to a mate, a defense mechanism to avoid more dominant individuals, or a survival strategy. It can also be a physical characteristic that establishes an individual's place in society. Sexual mimicry is employed differently across species and it is part of their strategy for survival and reproduction. Examples of sexual mimicry in animals include the spotted hyena, certain types of fish, passerine birds and some species of insect among others. These are cases of intraspecific sexual mimicry, but interspecific sexual mimicry can also occur in some plant species, especially orchids. In plants employing sexual mimicry, flowers mimic mating signals of their pollinator insects. These insects are attracted and pollinate the flowers through pseudocopulations or other sexual behaviors performed on the flower.
Social systems
Sexual mimicry can influence the species’ social system. The most common example is the spotted hyenas, Crocuta crocuta. Female hyenas resemble male hyenas in their sexual anatomy: the females have peniform clitorises, resembling a penis, and false scrotal sacs. These characteristics, as well as high androgen levels in their blood, make for aggressive females, which results in their dominance over males; the female with the lowest rank is more dominant than the highest-ranking male. Within the female population in each clan, there are different ranks: the dominant females, who reproduce at an earlier age and get more access to food, and the non-dominant females. Their dominance is hierarchical and is passed from mother to daughter. By contrast, male spotted hyenas gain their social status with the length of their stay in the clan;[1] it does not involve aggressive contests. The males leave their clan between the ages of two and six [2] and join a different clan where they gain status with age. Males also foster amicable relationships with the females to stabilize their position in the social hierarchy.[3]

Because females are the dominant sex among spotted hyenas, they are the most respected. Subordinate female hyenas initiate a ‘greeting’ with dominant female hyenas as a sign of respect and are forced to do so if they refuse. This greeting used by hyenas reflects the asymmetry of their ranking; the animal being greeted (the subordinate individual) extends its hind legs and the individual doing the greeting (the dominant hyena) licks or sniffs the erect peniform clitoris.[3] By lifting its hind legs, the hyena being greeted (the subordinate hyena) exposes its most vulnerable body part to the other individual, an act that reflects inferiority. As well, when its hind legs are lifted, a scent can be identified by the other individual. Subordinate hyenas expose their scent more often than high-ranking hyenas. This greeting, however, is not commonly seen between males and adult females; when it does occur, it is restricted to males of median or higher rank greeting dominant females.[3]
Mating systems
In the spotted hyenas, the only way for the males to mate with the females is if they have the female's full cooperation because of the female's peniform clitoris. An increase in the male's status gave them more access to dominant females in the clan. Female dominant hyenas do not mate with multiple males, possibly due to the cost of cleaning their genitalia, which hyenas are seen doing after copulation.[1] Because they will get access to the most dominant and better fit males, they do not need to copulate with multiple males to produce offspring of higher fitness. Non-dominant females are observed copulating more often with lower-ranking males.[3] It is costly for female hyenas to give birth through their long peniform clitoris. The umbilical cord is 12–18 cm long, while the journey from the uterus to the clitoris end is 40 cm. The umbilical cord often breaks before the cub emerges, leading to death by anoxia for many young. This journey is not only harmful for the cubs, but also for the mother. The tissue of the clitoris will sometimes rip open when giving birth for the first time which can be fatal to the mother.[4]
Female spotted hyenas are the choosy sex because they invest in parental care as well as being the dominant sex in the clan. However, males are likely to still have a preference for a particular female as it is seen in other animals; high-ranking females begin breeding at a younger age and their offspring are more likely to survive to adulthood than the offspring of low-ranking females. Males associate more closely with females that are fertile, a state most likely noticed through olfactory cues.[5] While middle/high-ranking males associate with high-ranking females, low-ranking males associate equally with high and low-ranking females. Associating with low-ranking females may be due to low-ranking males failing to recognize the reproductive success of high-ranking females or using a different type of reproductive strategy. Males tend to spend lot of time with the female they mate with before conception to avoid other males coming in close contact with her.[5]
Sexual mimicry is also used as a mate-guarding strategy by some species. Mate-guarding is a process in which a member of a species prevents another member of the same species from mating with their partner. Mate-guarding is seen in Cotesia rubecula, a parasitic wasp from the family Braconidae whose mating system is polygynous. Males are attracted to females through pheromones and they induce females to mate through vibrations, to which the female responds by assuming a specific position. When a male who has copulated with a female sees another male trying to court her, he will often adopt the female receptive position. Post-copulatory female mimicry by the male offers an advantage by acting as a mate-guarding mechanism. If a second male arrives soon enough after the female copulates with the first male, the second male may be able to induce a second copulation which will compete with the first one. However, if the first male who copulated with her mimics the female, it distracts the second male long enough that the female becomes unreceptive.[6]
Sneaky copulation


Sneaky copulation is a strategy used by many aquatic organisms who portray sexual mimicry. Several studies have found that small male fish will look and behave like the female of their species in order to gain access to female territory and copulate with them.[7][8] In the fish family Blenniidae, the female Salaria pavo will show a specific colour pattern and movement when they want to approach a male and copulate with him. The male guards a territory, and when the female lays her eggs, the parental male protects that territory until the eggs hatch. A second type of males, the sneaker males, is parasitic and resembles the female bleniid fish in their small size, colour, and movement patterns. This allows them to intrude into the nest guarded by the parental males. Sneaker males approach the nests with the same colour patterns and movements that the females hold. Most cases of sneaker males are seen when there is a female already inside the nest although sometimes the sneaker fish enters the nest alongside a female. This species of fish releases the sperm before the female releases her eggs into the water[9] making it possible for the sneaker fish to fertilize an egg, even if the female is not present in the nest.[7]

In the Sepiina family, Sepia apama, also known as cuttlefish, have some males that are large and able to guard a female's nest while other males are small and resemble females in order to sneak in copulations. In the giant cuttlefish, the male courts the female and transfers its sperm to a pouch below the female's beak. During this process, the female displays a body pattern of black splotches on a white background. Once the eggs are laid, the male guards the nest from any possible suitors and opponents. A ‘second female’ is sometimes seen during male-female interaction in close proximity to the couple. This female-looking cuttlefish has the same black blotches as a real female. If the male leaves to fight other males, this individual approaches the female and copulates with her, usually with success. However, in the absence of rivals, these 'mimicking female' males display the phenotype of a mature male.[8]
Sexual mimicry against aggression
A similar phenomenon to the sneaker fish males is observed in the dark-edged splitfin, Girardinichthys multiradiatus. The juveniles resemble the pregnant females in the species by having a dark spot near the vent. In this case, however, the mimicking males have the capability to resemble the females or become a morphologically mature male throughout most of their adult life. This dark spot allows the female-looking males to escape aggression from more dominant males, as well as reducing the chance of having a female nearby flee due to persisting courting males. The mature males do not attack the subordinate fish and the subordinate fish decides when to initiate the fights, which gives it an advantage as the mature male is not expecting this. The dark spot also permits access of subordinate males to females, a characteristic that is advantageous because females’ eggs can only be fertilized during a five-day fertilizing window.[10]


Sexual mimicry to avoid aggression is also seen in birds. In some bird species, males have a female-like plumage colour during their second year of life (SY males). These SY males are sexually mature and able to breed, but their morphology differs greatly from the older, after second year (ASY) males. Various studies have looked into this delayed plumage maturation (DPM) and found that the DPM in SY males reduces aggression from ASY males.[11][12][13] Female mimicry in birds was first found in European-pied flycatcher, Ficedula hypoleuca. When a dull-coloured male is in the area, mature males reduce their aggressiveness and behave as if the intruder is a female. The dull plumage is seen mostly in younger males, likely due to being born later in the previous spring. The resemblance to females benefit these young males when trying to occupy a territory with many males already present because the young males can gain information and access to a territory that would not be accessible to them otherwise.[12]
There is a big cost to not looking like a male when it comes to defending a territory or attracting a mate. Females show aggression against dull-coloured males, making it harder for them to mate.[12] However, DPM has some benefits: as mentioned above, it reduces aggression from older males.[12] As well, these female-looking birds are able to get access to territories, mates, and food that may be not be available to them otherwise. Another benefit is that DPM provides SY birds with a longer lifespan; because they do not have to compete with other males, their mortality rate is lower. This advantage, however, only benefits individuals of species that have a longer potential lifespan and, therefore, DPM would not benefit a short-lived species.[11] This is known as the breeding threshold hypothesis, and states that SY males should only delay breeding if there is a large mortality difference between the SY males who attempt to breed and the ones who do not.[11]

Most studies addressed DPM as a type of sexual mimicry,[11][12] which is done through deception: male ASY birds should not be able to tell females or SY males apart. However, Muheter et al. (1997) found that territorial males perceive the dull-coloured males as males but they show less aggression because their dull-coloured plumage promotes low competitive ability. They referred to this as honest signalling and not sexual mimicry.[13]
Another example of sexual mimicry occurs in Broadley's Flat Lizard, Platysaurus broadleyi, where some males mimic females. Flat lizard males tend to be territorial and aggressive towards other males. Therefore, it is beneficial for some males to mimic females in order to avoid aggressive encounters and move freely through the male's territory, looking for mates. There are two types of males in this population; she-males, who mimic females, and he-males, who look like males. The she-males can visually fool the he-males into believing that they are female due to their female morphology. However, the she-males cannot fool the he-males through scent, as he-males can detect the difference. Therefore, the most successful she-males are those who avoid close contact with other males, thereby reducing the chances of detection through chemical signals.[14]
Molecular control over sexual mimicry
Female hyenas’ sexual mimicry to males is part of their anatomy and it is thought to have evolved through high androgen levels. While female ancestors were smaller than males, selection must have acted upon androgen levels and female body size to increase both, leading to further selection and larger females than males.[15] The high androgen levels are not present in the female ovaries, as it was once thought;[16] the stromal tissue in the ovaries contains lower testosterone levels than the males’ testes. However, females’ androgen levels in the blood are as high as the ones found in the male, having the effect of morphologically male-looking females.[17]
Ruffs can also show sexual mimicry through a combination of genetics and hormones. In a population of ruffs, Philomachus pugnax, there are three types of male morphs: independent males and satellite males, both of which are reproductive competitors, and faeder ruffs that resemble females in their plumage. The first two morphs are controlled by a dominant allele at a single autosomal locus, while the third morph is likely to have come from a combination of a third allele and a lack of testosterone.[18] When testosterone is administered to reeves (female ruffs), male courtship behaviour and male feather colouration are expressed in the reeves. Testosterone, in this case, expresses sex-limited characteristics by acting on the single autosomal gene.[19] Similarly, while it has not yet been tested, it is likely that the lack of testosterone is the cause for the faeder ruffs’ similarity to females.
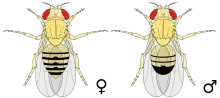
A different example is seen in mature female fruit flies, Drosophila melanogaster, who are very attractive but their level of attractiveness decreases by half or more after three minutes of mating.[20] Males release a compound, 7-tricosene, into the female during courtship that lowers female attractiveness. However, the researcher found that the females release this compound as well, six hours after mating. This compound lowers the female's levels of attractiveness both times, when the male is courting her and during mating. This way, the female mimics the male and with this compound, she lowers her levels of attractiveness.[21]
Genetic control over sexual mimicry
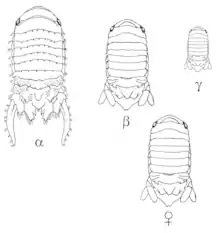
Some organisms’ sexual mimicry is genetically determined by specific alleles. Unlike sexual mimicry that arises due to molecular compounds or hormones and can sometimes be induced through these molecules, this sexual mimicry arises from the organism's genetic material. Besides the female hyenas’ sexual anatomy, which is part of their genetics, some other organisms have only some males/females in their population who look like the opposite sex and this is determined by specific alleles.
In the marine isopod population, Paracerceis sculpta, there are three different male morphologies: the alpha male is the largest morph, it matures last, and it is the one who gets privileged access to the females. The beta male is of intermediate size, and it mimics the female to get access to females. Last, the gamma male is the smallest morph and it invades harems, where females go to mate with alpha males, for mating opportunities. This morphology is associated with a single autosomal gene and three different alleles. Beta is the most dominant allele, followed by gamma, which is followed by alpha. Selection on these alleles acts according to the Hardy-Weinberg equilibrium and mating success is equivalent among all three morphs.[22]
The alpha males, who are homozygous for the alpha allele, mate with many females in a harem. The females prefer to aggregate with other females in the harem, which gives the alpha male a bigger selection of mating partners. Shuster (1992) looked at the behaviour and relationship of each morph with respect to the harem and found that beta and gamma males could locate harems that have sexually receptive females. They were also able to differentiate between a harem with a sexually receptive female, i.e. one that is able to mate, and a non-sexually receptive female, i.e. one that has already deposited the embryo into her pouch and can no longer mate. While it is still unclear how the beta males do this or how their mating strategies work, they are not harassed by alpha males due to their mimicry of females: the beta males can attract other females into the harem since females like to go where other females are, and this provides the alpha males with more mates.[23]
Another order of organisms whose sexual mimicry is influenced by their DNA is the Odonata, carnivorous insects known as dragonflies and damselflies. In these species, it is the female who sometimes mimics the male. Within a species, groups of females will differ in colour: one group mimics the males’ colour and they are known as androchromes. Other groups will have their own female colouration and they are known as gynochromes. In Ischnura elegans, androchromes comprise 6-30% of the female population and their colour is usually blue, like the males; in some populations, androchromes are larger in size than gynochromes. This polymorphism is controlled by an autosomal allele and some studies have looked at the reason for the polymorphism's maintenance.[24]


The most likely theory for the maintenance of the polymorphism in Odonata is the density dependence theory [25] that states that at a high male density, the androchromes are not bothered by the males and their existence is not threatened by male harassment. This hypothesis also assumes that males cannot distinguish between androchromes and other males. This advantage, however, is counteracted with the fact that they will not get a lot of mating opportunities (if any) and their reproduction is limited. This theory is the most likely explanation for the maintenance of polymorphism, since studies have shown that there is an advantage for androchromes in high male-density populations.[26]
Self-control over sexual mimicry
While, as seen before, most organisms which portray sexual mimicry are born with this morphology/behaviour, this is not always the case. The giant cuttlefish, Sepia apama, mentioned above in the section “sneaky copulations”, is born with the capacity to choose whether to change its morphology to look like a female or a mature male. When no competition is seen nearby, the cuttlefish will look like a mature male and mate with the female. However, when a mature male and a female are copulating, the giant cuttlefish will resemble a female and stay at a close distance of the couple, hoping for a chance to mate with the female if the mature male leaves to fight other males.[8] Another example of an organism that has the capability to remain small and look like a female, or become a morphologically mature male, is the dark-edged splitfin, Girardinichthys multiradiatus. The purpose for their female mimicry was seen before, in the “sexual mimicry against aggression” section where the female-looking males will escape aggression from dominant males and avoid females fleeing their company due to persisting courting males.[10]
Interspecific deceptive mimicry

Interspecific sexual mimicry can also occur in some plant species. The most common example of this is known as sexually deceptive pollination and is found among some orchids.[27] The orchid mimics its pollinator's females, usually hymenopterans such as wasps and bees, attracting the males to the flower. Orchid flowers mimic the sex pheromones and to some degree the visual appearance of the female insect of its pollinator species. The primacy of olfactory over visual cues has been demonstrated in many cases, such as in the European orchid genus Ophrys as well as many Australian sexually deceptive orchids. In few other cases, such as the South African daisy Gorteria diffusa, visual signals seem to be of primary importance.[28] Visual signals also enhance the attractiveness of the flowers of some Ophrys species to their pollinators.[29][30] Some male scoliid wasps such as Campsoscolia ciliata are more attracted to the Ophrys flowers’ odours than to the odours of the female wasps, although they both attract the males with the same compounds. This is most likely a result of a higher amount of scent coming from the orchid flowers; female wasps tend to produce less scent to avoid attracting predators.[31] Regardless of whether orchids use appearances, fragrances or both, they mimic the female pollinator for their own benefit.
References
- East ML (1 September 2001). "Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females". Behavioral Ecology. 12 (5): 558–568. doi:10.1093/beheco/12.5.558.
- Smale L, Nunes S, Holekamp KE (1997). "Sexually dimorphic dispersal in mammals: patterns, causes and consequences". In Slater PJ, Rosenblatt JS, Snowdon CT, Milinski M (eds.). Advances in the Study Behavior. 26. Academic Press. pp. 181–250. ISBN 9780080582870.
- East ML, Burke T, Wilhelm K, Creig C, Hofer H (2003). "Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure". Proceedings of the Royal Society B. 270 (1521): 1247–54. doi:10.1098/rspb.2003.2363. PMC 1691369. PMID 12816637.
- Frank LG (February 1997). "Evolution of genital masculinization: why do female hyaenas have such a large 'penis'?". Trends in Ecology & Evolution. 12 (2): 58–62. doi:10.1016/S0169-5347(96)10063-X. PMID 21237973.
- Szykman M, Engh AL, Horn RC, Funk SM, Scribner KT, Holekamp KE (2001). "Association Patterns among Male and Female Spotted Hyenas (Crocuta crocuta) Reflect Male Mate Choice". Behavioral Ecology and Sociobiology. 50 (3): 231–238. doi:10.1007/s002650100356. JSTOR 4601958.
- Field SA, Keller MA (December 1993). "Alternative mating tactics and female mimicry as post-copulatory mate-guarding behaviour in the parasitic wasp Cotesia rubecula". Animal Behaviour. 46 (6): 1183–1189. doi:10.1006/anbe.1993.1308.
- Gonçalves EJ, Almada VC, Oliveira RF, Santos AJ (11 May 2009). "Female Mimicry as a Mating Tactic in Males of the Blenniid Fish Salaria Pavo". Journal of the Marine Biological Association of the United Kingdom. 76 (2): 529. doi:10.1017/S0025315400030721.
- Norman MD, Finn J, Tregenza T (7 July 1999). "Female impersonation as an alternative reproductive strategy in giant cuttlefish". Proceedings of the Royal Society B. 266 (1426): 1347–1349. doi:10.1098/rspb.1999.0786. PMC 1690068.
- Patzner RA (1984). "The reproduction of Blennius pavo (Teleostei Bleniidae). II. Surface structure of the ripe egg". Zoologischer Anzeiger. 213: 44–50.
- Macías-Garcia C, Valero A (19 May 2010). "Context-dependent sexual mimicry in the viviparous fish". Ethology Ecology & Evolution. 13 (4): 331–339. doi:10.1080/08927014.2001.9522764.
- Studd MV, Robertson RJ (1985). "Life Span, Competition, and Delayed Plumage Maturation in Male Passerines: The Breeding Threshold Hypothesis". The American Naturalist. 126 (1): 101–115. doi:10.1086/284399. JSTOR 2461565.
- Slagsvold T, Saetre G (June 1991). "Evolution of Plumage Color in Male Pied Flycatchers (Ficedula Hyopleuca): Evidence for Female Mimicry". Evolution. 45 (4): 910–917. doi:10.1111/j.1558-5646.1991.tb04359.x. PMID 28564056.
- Muehter VR, Greene E, Ratcliffe L (27 October 1997). "Delayed plumage maturation in Lazuli buntings: tests of the female mimicry and status signalling hypotheses". Behavioral Ecology and Sociobiology. 41 (4): 281–290. doi:10.1007/s002650050389.
- Whiting MJ, Webb JK, Keogh JS (25 February 2009). "Flat lizard female mimics use sexual deception in visual but not chemical signals". Proceedings of the Royal Society B. 276 (1662): 1585–1591. doi:10.1098/rspb.2008.1822. PMC 2660994. PMID 19324828.
- Hamilton WJ 3rd, Tilson RL, Frank LG (26 April 2010). "Sexual Monomorphism in Spotted Hyenas, Crocuta crocuta". Ethology. 71 (1): 63–73. doi:10.1111/j.1439-0310.1986.tb00570.x.
- Matthews LH (5 July 1939). "Reproduction in the Spotted Hyaena, Crocuta crocuta (Erxleben)". Philosophical Transactions of the Royal Society B. 230 (565): 1–78. doi:10.1098/rstb.1939.0004.
- Racey PA, Skinner JD (20 August 2009). "Endocrine aspects of sexual mimicry in Spotted hyaenas Crocuta crocuta". Journal of Zoology. 187 (3): 315–326. doi:10.1111/j.1469-7998.1979.tb03372.x.
- Lank DB, Farrell LL, Burke T, Piersma T, McRae SB (6 November 2013). "A dominant allele controls development into female mimic male and diminutive female ruffs". Biology Letters. 9 (6): 20130653. doi:10.1098/rsbl.2013.0653. PMC 3871350. PMID 24196515.
- Lank DB, Coupe M, Wynne-Edwards KE (22 November 1999). "Testosterone-induced male traits in female ruffs (Philomachus pugnax): autosomal inheritance and gender differentiation". Proceedings of the Royal Society B. 266 (1435): 2323–2330. doi:10.1098/rspb.1999.0926. PMC 1690456.
- Tompkins L, Hall JC (January 1981). "The different effects on courtship of volatile compounds from mated and virgin Drosophila females". Journal of Insect Physiology. 27 (1): 17–21. doi:10.1016/0022-1910(81)90026-3.
- Scott D (November 1986). "Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 83 (21): 8429–33. doi:10.1073/pnas.83.21.8429. PMC 386942. PMID 3095835.
- Shuster SM, Wade MJ (18 April 1991). "Equal mating success among male reproductive strategies in a marine isopod". Nature. 350 (6319): 608–610. doi:10.1038/350608a0.
- Shuster SM (1992). "The Reproductive Behaviour of α-, β-, and γ-Male Morphs in Paracerceis sculpta, a Marine Isopod Crustacean" (PDF). Behaviour. 121 (3/4): 231–258. doi:10.1163/156853992X00381. JSTOR 4535029.
- Cordero A, Carbone SS, Utzeri C (January 1998). "Mating opportunities and mating costs are reduced in androchrome female damselflies, Ischnura elegans(Odonata)". Animal Behaviour. 55 (1): 185–197. doi:10.1006/anbe.1997.0603. PMID 9480685.
- Hinnekint BO. 1987. Population dynamics of Ischnura e. Elegans (Vnader Linden)(Insecta:Odonata) with special reference to morphological colour changes, female polymorphism, multiannual cycles and their influence on behaviour. Hydobiologia. 146: 3-31.
- Cordero A, Andres JA (1996). "Colour polymorphism in odonates: females that mimic males?". Journal of the British Dragonfly Society . 12 (2): 50–60.
- Schiestl FP (1 June 2005). "On the success of a swindle: pollination by deception in orchids". Naturwissenschaften. 92 (6): 255–264. doi:10.1007/s00114-005-0636-y. hdl:20.500.11850/32223. PMID 15931514.
- Ellis AG, Johnson SD (November 2010). "Floral Mimicry Enhances Pollen Export: The Evolution of Pollination by Sexual Deceit Outside of the Orchidaceae". The American Naturalist. 176 (5): E143–E151. doi:10.1086/656487. PMID 20843263.
- Gaskett AC, Herberstein ME (2 October 2009). "Colour mimicry and sexual deception by Tongue orchids (Cryptostylis)". Naturwissenschaften. 97 (1): 97–102. doi:10.1007/s00114-009-0611-0. PMID 19798479.
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Amaral Mdo C (June 2004). "Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae)". Annals of Botany. 93 (6): 755–62. doi:10.1093/aob/mch091. PMC 4242296. PMID 15051623.
- Ayasse M, Schiestl FP, Paulus HF, Ibarra F, Francke W (7 March 2003). "Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals". Proceedings of the Royal Society B. 270 (1514): 517–522. doi:10.1098/rspb.2002.2271. PMC 1691269. PMID 12641907.