Sudan III
Sudan III is a lysochrome (fat-soluble dye) diazo dye. It is structurally related to azobenzene.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
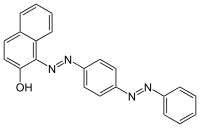
1-(4-(Phenyldiazenyl)phenyl)azonaphthalen-2-ol | |
| Other names
Sudan Red BK, Fat Ponceau G, Cerasin Red, C.I. 26100, Solvent Red 23, Sudan Red, Sudan Red III, Sudan V, Sudan Red B, Sudan G, Scarlet B, and Tony Red | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.490 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H16N4O | |
| Molar mass | 352.397 g·mol−1 |
| Melting point | 199 °C (390 °F; 472 K) |
| Hazards[1] | |
| Safety data sheet | Sigma-Aldrich Sudan III |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H315, H318, H319, H335, H413 | |
| P101, P102, P103, P270, P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P310, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
It is used to color nonpolar substances such as oils, fats, waxes, greases, various hydrocarbon products, and acrylic emulsions. Its main use is as a fuel dye in the United States of America mandated by the Internal Revenue Service (IRS) to distinguish low-taxed heating oil from automotive diesel fuel, and by the United States Environmental Protection Agency (EPA) to mark fuels with higher sulfur content; it is a replacement for Solvent Red 26 with better solubility in hydrocarbons.[3] The concentration required by IRS is a spectral equivalent of 3.9 pounds per 1000 barrels, or 11.13 mg/l, of Solvent Red 26 in solid form; the concentrations required by EPA are roughly 5 times lower.
Biological staining
Sudan III is a dye used for Sudan staining. Similar dyes include Oil Red O, Sudan IV, and Sudan Black B. They are used for staining of triglycerides in frozen sections, and some protein bound lipids and lipoproteins on paraffin sections. It has the appearance of reddish brown crystals and a maximum absorption at 507(304) nm.[4]
Safety
Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer.[5]
References
- "Safety Data Sheet Sudan III". fishersci.com. Retrieved 24 August 2020.
- Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245.
- "Archived copy". Archived from the original on 2005-05-10. Retrieved 2006-02-02.CS1 maint: archived copy as title (link)
- R. D. Lillie. Conn's Biological Stains. Williams & Wilkins, Baltimore, MD., U.S.A.
- Refat NA, Ibrahim ZS, Moustafa GG, Sakamoto KQ, Ishizuka M, Fujita S (2008). "The induction of cytochrome P450 1A1 by Sudan dyes". J. Biochem. Mol. Toxicol. 22 (2): 77–84. doi:10.1002/jbt.20220. PMID 18418879. S2CID 206010951.
- Susan Budavari, Editor, (1996). The Merck Index, Ed. 12. Merck & Co., Inc., Whitehouse Station, NJ, USA
- Edward Gurr, (1971). Synthetic Dyes in Biology, Medicine and Chemistry. Academic Press, London, England.
External links
- Stains File entry