TMEM106C
TMEM106C is a gene that encodes the transmembrane protein 106C (TMEM106C) in Homo sapiens It has been found to be overexpressed in cancer cells and also is related to distal arthrogryposis,[5][6] a condition of stiff joints and irregular muscle development. The TMEM106C gene contains a domain of unknown function, DUF1356, that spans most of the protein. Transmembrane protein 106C also goes by the aliases MGC5576 or MGC111210, LOC79022.[7]
| TMEM106C | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | TMEM106C, transmembrane protein 106C | ||||||||||||||||||||||||
| External IDs | MGI: 1196384 HomoloGene: 11440 GeneCards: TMEM106C | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) |
| ||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 12: 47.96 – 47.97 Mb | Chr 15: 97.96 – 97.97 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Location and gene neighborhood
The TMEM106C gene is located on the long arm of the 12th chromosome. It is found at position 12q13.1. This gene spans from 48357225 to 48362667 on chromosome 12.[7] This gene is in between COL2A1, the human type II collagen gene, and VDR, the human Vitamin D Receptor gene.[8] This protein is found to be an integral part of the endoplasm reticulum membrane.[9]
Protein structure
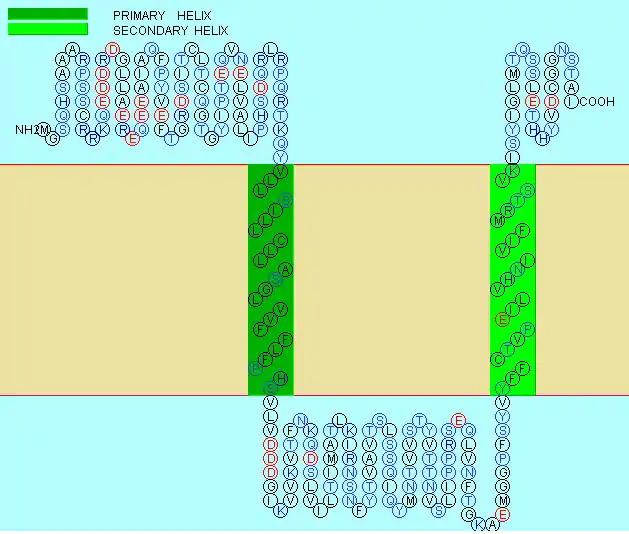
The TMEM106A protein has a molecular weight of 27.9 kdal with a PI of 6.325.[11] It has 250 amino acids, 230 of which are in the domain of unknown function. No signal peptide has been found for this protein but TMEM106C has transmembrane regions which gives evidence for an internal signal peptide.[12] This protein spans the ER membrane 2 times.[10] There is evidence that these transmembrane regions take on helical structures.[13] The predicted structure of the protein is shown to the left:
TMEM106C is valine-rich with no tryptophan.[11]
There are several areas for post-translational modification for TMEM106A including:[14]
- Phosphorylation[15]
- Kinase-Specific Phosphoylation[16]
- N-glycosylation[17]
Expression
This gene is highly expressed. TMEM106C is expressed 4.9 times the average gene.[7] TMEM106C has ubiquitous expression. It can be found expressed in many tissues types. Tissue types with high expression included the adrenal gland, eye, reproductive organs, cervix and blood. High expression was found using EST and GEO data.

This gene is also found overexpressed in cancer cells. This gene has found to be expressed three times more in adrenal tumor and twice more in bladder carcinoma and retinoblastoma than normal expression.

It is also found to be highly expressed in breast (mammary gland) tumor, cervical tumor, esophageal tumor, leukemia, liver tumor; lung tumor, pancreatic tumor, prostate cancer, and soft tissue/muscle tissue tumor.[20]
TMEM106C is found in all stages of development from embryoid body, blastocyst, fetus, infant, juvenile and adult.[21]
Homology
Paralogs
There are two paralogs for TMEM106C. These paralogs are TMEM106A and TMEM106B.[6] Both genes are found highly conserved in Mammalia. TMEM106A is also found to be conserved in invertebrates as well. The protein was found in tapeworms and other invertebrate worms.[22]
| Protein | Accession number | Amino acids | Identity percent |
|---|---|---|---|
| TMEM106A | AAI46977 | 262 | 36 |
| TMEM106B | NP_001127704 | 274 | 43 |
| TMEM106C | NP_001137314.1 | 250 | 100 |
Orthologs
TMEM106C is highly conserved in Mammalia. Links to sequences can be found in the table below:[22]
| Organism | Common name | Accession number | Amino acids | Identity percent | Notes |
|---|---|---|---|---|---|
| Homo sapiens | Human | NP_001137314.1 | 250 | 100 | Mammal |
| Macaca mulatta | Rhesus macaque | NP_001253653.1 | 249 | 98 | Mammal |
| Equus caballus | Horse | XP_001490277.1 | 249 | 90 | Mammal |
| Mus musculus | Mouse | NP_001239082.1 | 260 | 79 | Mammal |
| Alligator mississippiensis | American alligator | XP_006273403.1 | 271 | 77 | Reptile |
| Chrysemys picta bellii | Painted turtle | XP_005291963.1 | 270 | 73 | Reptile |
| Falco cherrug | Saker falcon | XP_005436184.1 | 274 | 74 | Aves |
| Gallus gallus | Chicken | XP_003643471.1 | 253 | 65 | Aves |
| Xenopus tropicalis | Western clawed frog | NP_001016848.1 | 263 | 64 | Amphibia |
| Latimeria chalumnae | Coelacanth | XP_005986345.1 | 258 | 61 | Actinoterygii |
| Danio rerio | Zebrafish | NP_001070764.1 | 275 | 57 | Actinoterygii |
References
- GRCh38: Ensembl release 89: ENSG00000134291 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000052369 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Genini, S. (16 May 2006). "Radiation Hybrid Mapping of 18 Positional and Physiological Candidate Genes for Arthrogryposis Multiplex Congenita on Porcine Chromosome 5". Animal Genetics. 37 (3): 239–244. doi:10.1111/j.1365-2052.2006.01447.x. PMID 16734683.
- "Genecards". The Human Gene Compendium.
- Thierry-Mieg, Danielle and Jean (2 Jun 2010). "Homo sapiens complex locus TMEM106C, encoding transmembrane protein 106C". Aceview National Center for BioInformation technology, National Library of Medicine, National Institutes of Health.
- "TMEM106C transmembrane protein 106C Homo sapiens (human)". NCBI. 4 May 2014.
- Nakai, K. (19 Nov 1999). "PSORT II Prediction".
- Mitaku Group. "Classification and Secondary Structure Prediction of Membrane Proteins". Department of Applied Physics Nagoya University.
- "Biology Workbench 3.2". SDSC: San Diego SuperComputer Center. 11 May 2011.
- Krogh, Anders (12 Jun 2013). "TMHMM Server v 2.0".
- Tusnady, G.E. (2001). "HMMTop: Prediction of transmembrane helices and topology of proteins v 2.0".
- Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H (2012). "ExPASy: SIB bioinformatics resource portal". Nucleic Acids Research. Nucleic Acids Res, 40(W1):W597-W603. 40 (Web Server issue): W597-603. doi:10.1093/nar/gks400. PMC 3394269. PMID 22661580.
- Blom N, Gammeltoft S, Brunak S (December 1999). "Sequence and structure-based prediction of eukaryotic protein phosphorylation sites". Journal of Molecular Biology. 294 (5): 1351–62. doi:10.1006/jmbi.1999.3310. PMID 10600390.
- Yu Xue; Jian Ren; Xinjiao Gao; Changjiang Jin; Longping Wen & Xuebiao Yao (10 Aug 2012). "Tool to Predict Kinase-specific Phosphorylation Sites in Hierarchy". GPS.
- R. Gupta, E. Jung & S. Brunak. (2004). "Prediction of N-glycosylation sites in human proteins". NetNGlyc 1.0 Server.
- "GEO data".
- "GEO data 2".
- Mosca E, Alfieri R, Merelli I, Viti F, Calabria A, Milanesi L (3 Jul 2010). "TMEM106C". Genes to Systems Breast Cancer Database.
- "TMEM106C: Transmembrane protein 106C". EST profile. First Gov. Health and Human Services. 28 Oct 2009.
- "BLAST". National Center for Biotechnology Information. National Library of Medicine. 2014.



