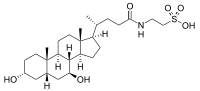
Tauroursodeoxycholic acid
Tauroursodeoxycholic acid (TUDCA) is an ambiphilic bile acid. It is the taurine conjugate form of ursodeoxycholic acid (UDCA). Humans are found to have trace amounts of TUDCA. However, bears contain large amounts of TUDCA in their bile; UDCA and conjugates comprise about 47% of the bile in American black bears and up to 76% in Asiatic bears.[1] TUDCA has been used in ancient Asian pharmacopoeias for its supposed beneficial effects. UDCA is produced in several countries for the treatment of gallstones and liver cirrhosis. It is not approved by the Food and Drug Administration, in the U.S. while UDCA is approved in the United States for the treatment of primary biliary cirrhosis[1][2] Ongoing research is finding TUDCA has diminishing apoptotic effects, with potential application in heart disease, Huntington's disease, Parkinson's disease, and stroke.[3][4] Recently, TUDCA has been found to have protective effects in the eye, especially concerning retinal degenerative disorders.
 | |
| Names | |
|---|---|
| IUPAC name
2-[[(4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino] ethanesulfonic acid | |
| Other names
TUDCA; 3α,7β-Dihydroxy-5β-cholanoyltaurine; UR 906; Ursodeoxycholyltaurine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H45NO6S | |
| Molar mass | 499.71 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Chinese medicine has used animal bile for hundreds of years as a medicine to treat "heat" illnesses. It was used to relieve spasms, reduce fever, and improve visual acuity. Bile is naturally synthesized via cholesterol, consisting of compounds including taurochenodeoxycholic acid, ursodeoxycholic acid, and chenodeoxycholic acid.[5] However, UDCA and TUDCA were first synthetically developed in 1954 in Japan.[4]
Cellular mechanism
Apoptosis, or programmed cell death, is largely influenced by the mitochondria. If the mitochondria are distressed, they release the molecule cytochrome C (cyC). Cytochrome C initiates enzymes called caspases to propagate a cascade of cellular mechanisms to cause apoptosis. TUDCA prevents apoptosis with its role in the BAX pathway. BAX, a molecule that is translocated to the mitochondria to release cytochrome C, initiates the cellular pathway of apoptosis.[4] TUDCA prevents BAX from being transported to the mitochondria. This protects the mitochondria from perturbation and the activation of caspases. [6]
TUDCA also acts as a chemical chaperone.
Current research
Studies in recent years are continually showing ocular protective effects via TUDCA.
Photoreceptor cells
A study examined the effects of TUDCA on cones, in relation to retinitis pigmentosa (RP), a disease in which retinal rods and cones undergo apoptosis. Mice models were used, a wild-type and a mutant RP model, rd10. Both models were injected with TUDCA every 3 days from post-natal day 6 (p6) to p30 and compared to the vehicle. Electroretinography (ERG), photoreceptor cell counts, cone photoreceptor nuclei counts, and TUNEL labeling were all analyzed to show the effects of TUDCA. The dark-adapted and light-adapted ERG responses were greater in the TUDCA treated mouse than the vehicle treated mouse. TUDCA treated mice also had more photoreceptor counts, yet non-altered retinal morphology or function. Even at P30, a stage where rod and cone function is usually greatly diminished in the rd10 mouse model, the photoreceptor function was protected.[7]
Another study, from the Department of Ophthalmology at Johns Hopkins University, in Baltimore, Maryland, saw similar effects in two components of bile, bilirubin and TUDCA, in relation to RP. Oxidative stress and prolonged light exposure were studied in rd10 mice and albino mice. In rd10 mice, intraperitoneal injections of bilirubin or TUDCA were given every 3 days starting at P6. This caused a considerable preservation in cone cell amount and function at P50, and a modest rod cell amount at P30. In the albino mice models, intraperitoneal injections of bilirubin or TUDCA were given prior to prolonged light exposure. Both treatments had positive effects on the health of the mouse retina, including a reduced accumulation of superoxide radicals, rod cell death, and disruption of cone inner and outer segments. The findings of the study are elucidating optimized conditions for RP treatment[8]
Choroidal neovascularization
A study done at the Department of Ophthalmology at Seoul National University College of Medicine examined the effects of TUDCA and UDCA on laser-treated choroids of rat models. Argon lasers were used to induce choroidal neovascularization (CNV) in rat models. TUDCA and UDCA were injected intraperitoneally 24 hours before and daily after the laser treatment. Fourteen days after laser-treatment, the eyes were examined for effects. Fluorescein angiography showed lower leakage from the CNV in UDCA and TUDCA treated groups than the control group. Additionally, vascular endothelial growth factor (VEGF) levels in the retina were examined and showed lower levels in the TUDCA treated group compared to the control group, whereas no effect in the UDCA treated group. TUDCA and UDCA may suppress CNV formation, which may be associated with its anti-inflammatory effects.[9]
Synaptic connectivity
A study from the Department of Physiology in University of Alicante, in Alicante, Spain, shows the effects of TUDCA in the P23H transgenic rat, a model of autosomal dominant retinitis pigmentosa. The transgenic rats were injected with TUDCA once a week starting from P21 until P120, along with vehicle-administered controls. At P120, the functionality of the retina was examined via ERG and immunoflourescent microscopy. The amplitude of the a- and b- waves were considerably higher in TUDCA treated rats, compared to the control group. Photoreceptor density in the center of the retina was three-fold greater in TUDCA treated rats. Also, TUNEL results showed smaller amounts of TUNEL-positive cells. The synaptic contacts amongst photoreceptor cells, bipolar cells, and horizontal cells were preserved in the TUDCA treated P23H rats. Additionally, the synaptic terminals in the outer plexiform layer were of greater density that in control rats. The neuroprotective effects of TUDCA are not only preserving retinal morphology and function, but also its synaptic contacts, a potentially useful aspect in delaying RP.[10]
See also
References
- Boatright, Jeffrey H.; Nickerson, John M.; Moring, Anisha G.; Pardue, Machelle T. (2009). "Bile acids in treatment of ocular disease". Journal of Ocular Biology, Diseases, and Informatics. 2 (3): 149–159. doi:10.1007/s12177-009-9030-x. PMC 2798994. PMID 20046852.
- Duan, WJ, Zhang, FK, Ou XJ, Zhang, T, Wang, XM, Wang, Y, Cui, Y, Zhao, XY, Jia, JD (2011). "[The clinical profiles of primary biliary cirrhosis with a suboptimal biochemical response to ursodeoxycholic acid]". Zhonghua Gan Zang Bing Za Zhi. 19 (2): 118–120. doi:10.3760/cma.j.issn.1007-3418.2011.02.011 (inactive 2021-01-11). PMID 21492515.CS1 maint: DOI inactive as of January 2021 (link)
- Vang, S; Longley, K; Steer, CJ; Low, WC (May 2014). "The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases". Global Advances in Health and Medicine. 3 (3): 58–69. doi:10.7453/gahmj.2014.017. PMC 4030606. PMID 24891994.
- Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC (2007). "Administration of Tauroursodeoxycholic Acid (TUDCA) Reduces Apoptosis Following Myocardial Infarction in Rat". The American Journal of Chinese Medicine. 35 (2): 279–295. doi:10.1142/S0192415X07004813. PMID 17436368. S2CID 28905884.
- Luo Q, Chen Q, Wu Y, Jiang M, Chen Z, Zhang X, Chen H (2010). "[Chemical constituents of bear bile]". Zhongguo Zhong Yao Za Zhi. 35 (18): 2416–2419. PMID 21141490.
- "TUDCA: Tauroursodeoxycholic Acid". stanford.edu. 2010-06-29.
- Phillips Joe M; Walker Tiffany A; Choi Hee-Young; Faulkner Amanda E; Kim Moon K; Sidney Sheree S; Boyd Amber P; Nickerson John M; Boatright Jeffrey H; Pardue Machelle T (2008). "Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30". Invest Ophthalmol Vis Sci. 49 (5): 2148–2155. doi:10.1167/iovs.07-1012. PMC 2626193. PMID 18436848.
- Oveson BC, Iwase T, Hackett SF, Lee SY, Usui S, Sedlak TW, Snyder SH, Campochiaro PA, Sung JU (2011). "Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration". J. Neurochem. 116 (1): 144–153. doi:10.1111/j.1471-4159.2010.07092.x. PMC 4083853. PMID 21054389.
- Woo SJ, Kim JH, Yu HG (2010). "Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model". J Ocul Pharmacol Ther. 26 (3): 223–229. doi:10.1089/jop.2010.0012. PMID 20565307.
- Fernández-Sánchez L, Lax P, Pinilla I, Martín-Nieto J, Cuenca N (2011). "Tauroursodeoxycholic Acid (TUDCA) Prevents Retinal Degeneration in Transgenic P23H Rats". Invest Ophthalmol Vis Sci. 52 (8): 4998–5008. doi:10.1167/iovs.11-7496. PMID 21508111.