Thioketal
In chemistry, a thioketal is the sulfur analogue of a ketal, with one of the oxygen replaced by sulfur. A dithioketal has both oxygens replaced by sulfur.
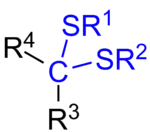
General chemical structure of a dithioketal
Thioketals can be obtained by reacting ketones or aldehydes with thiols.
Ketones can be reduced at neutral pH via conversion to thioketals; the thioketal prepared from the ketone can be easily reduced by catalytic hydrogenation using Raney nickel in a reaction known as the Mozingo reduction.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
