Torreya taxifolia
Torreya taxifolia, commonly known as the stinking-cedar or Florida torreya, but also sometimes as gopher wood or Florida nutmeg, is an endangered tree of the yew family, Taxaceae,[7][8] found in the Southeastern United States, at the state border region of northern Florida and southwestern Georgia.[1]
| Torreya taxifolia | |
|---|---|
 | |
| Leaves of Torreya taxifolia | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Division: | Pinophyta |
| Class: | Pinopsida |
| Order: | Pinales |
| Family: | Taxaceae |
| Genus: | Torreya |
| Species: | T. taxifolia |
| Binomial name | |
| Torreya taxifolia | |
 | |
| Native range | |
| Synonyms[2][3][4][5][6] | |
T. taxifolia became one of the first federally listed endangered plant species in the United States in 1984;[9][10] the IUCN has listed the species as critically endangered since 1998.[1][11] In 2010 98% of the mature trees of the species were believed to have been destroyed due to a poorly understood fungal blight as well as inundation due to dams and destruction by deer using trees as antler rubbing posts.[1][7]
Taxonomy
In 1821 the United States had managed to conquer the Florida Territory from Spain after years of surreptitiously trying to usurp Spanish rule, and begun the process of ethnically cleansing the land of its previous inhabitants. Plantation owners and their slaves began to move south to colonise and exploit the new land. One among them was the patriarch of the wealthy Croom family, who in 1826 purchased land around the town of Tallahassee. When he died in 1829, his two sons inherited his holdings and resolved to invest further in the region, buying up or leasing numerous plantations and eventually becoming the largest landowners in the area. They had all of their slaves shipped from their North Carolina plantations to labour at clearing the land, cultivating maize and cotton, and building giant mansions for their slave-masters. One of the two sons was Hardy Bryan Croom, who besides having studied law, being state senator in North Carolina since his early thirties, and his duties running the family and managing his properties, devoted some time to exploring the sciences.[12][13][14][15] Among other scientific interests,[14] he describes himself as fond of botany, had assembled a small personal herbarium,[16] and posthumously authored a monograph on the carnivorous plant genus Sarracenia.[14]
East across the Apalache River from the first estate of his brother Bryan Croom in Gadsden, who had acquired it in 1826 and coined it 'Rocky Comfort',[14][15] (another source maintains Hardy Bryan Croom leased the property in 1832), he noted that the flora was quite unique compared to elsewhere in the Tallahassee region,[15] and sent specimens to the herbaria in the north from 1833,[12][17] and corresponded with the botanist John Torrey about the plants here from 1834 onwards.[16] Among the exsiccata he sent north in 1833 were samples of a type of yew,[12] which in his 1834 first letter of reply to an inquiring Torrey he describes as having "little doubt" was the Taxus baccata, the common yew of Europe. His description of the red berries appears to confuse this tree with another rare, local and hitherto undescribed species, T. floridana.[16]
Torrey realised that Croom was quite mistaken, and that the specimens represented a species new to science.[17] Croom and his entire family drowned in a shipwreck off the coast of North Carolina in 1837.[12][13][14][15] The novel species was eventually described by George Arnott Walker-Arnott in April 1838 from specimens sent to Torrey and collected in Florida by Croom.[17][18]
The species was moved to the junior synonym Caryotaxus taxifolia in 1865 by Johann Baptist Henkel and Wilhelm Christian Hochstetter in their monograph on the conifers of the world, Synopsis der Nadelhölzer.[3] In 1873 Karl Heinrich Emil Koch moved the species to Foetataxus taxifolia.[19] In 1891 Edward Lee Greene validated Constantine Samuel Rafinesque-Schmaltz's generic epithet Tumion and erroneously moved this species there as Tumion taxifolium.[20][21]
In Thomas Nuttall's entry about Torreya taxifolia in his book about American trees, which was published in 1849 although it had been for the most part completed in 1841, he relates that in the correspondence Torrey had sent him, mention had been made of specimens of another species of taxoid tree which had been sent to him by Croom from the same region. To this plant Nuttall "doubtfully attaches the name" Taxus montana, somewhat of a nomen nudum, because Nuttall never actually described the plant besides quoting a summary description from Torrey's letter to him. Nuttall is doubtful about the taxon, because according to him it seems "scarcely distinct" from T. brevifolia of the Pacific Northwest.[17] Following the publication of this work, however, he was attributed as the author of this scientific name.[3][4] By 1865 this name was misapplied to Torreya taxifolia under the name Torreya montana. Henkel and Hochstetter synonymised this taxon with T. taxifolia in their work mentioned above.[3] According to the Index Kewensis this was in error; the name Taxus montana had actually already been given to a species, now Prumnopitys montana, described (validated, in fact) in 1806 by Carl Ludwig Willdenow from specimens collected by Alexander von Humboldt and Aimé Bonpland on their famous scientific exploration of the Americas, and Nuttall had in fact referred to Willdenow's species.[4] John Nelson, in his more utilitarian as opposed to scientific 1866 horticultural handbook of firs and pines for growing in Britain, introduced the name Foetataxus montana to write about Torreya taxifolia, apparently unaware of the German publication the previous year.[5][22] In fact, all these sources were wrong, for Nuttall states that he found a newer specimen of Croom's, of the same taxon, in the Herbarium of the Academy of Natural Sciences of Philadelphia labelled as Taxus floridana![17] Despite that the original synonymy with T. floridana, all these names are still maintained in the synonymy of Torreya taxifolia in some modern databases as of 2020.[2]
The University of North Carolina Herbarium has a single specimen, originally from the Jesup Herbarium of Dartmouth College, sent hither by Croom from the "Apalache River" in 1833. Curiously, it was first labelled as "Taxus montana Willd.", a South American tree, which was then later changed to Podocarpus taxifolia from southern New Zealand, and finally relabelled as Torreya taxifolia.[12]
Higher classification
It is the type species of the genus Torreya. The species, and the genus Torreya in general, has also been placed in the botanical family Cephalotaxaceae.[23]
Etymology
Arnott commemorated Torrey in the generic epithet.[24][17][25] The etymology of the specific epithet is from Latin taxus, meaning 'yew', and folium, meaning 'leaf': i.e., 'yew-leaved'.[26][27] Other species of Torreya have longer, less yew-like leaves, but this is not the reason that it was given this name, as the other species were described after this one.[26]
Common names
It is likely most often known locally by the common name stinking-cedar.[6][7][9][28][8][26][24][29][25] Another more common local name used is gopher wood.[1][6][7][9][28][26][29][25] Nuttall, writing in the early 1840s, coins the name "yew-leaved torreya" for it, but describes that in the land where it grows in the 1830s, it was known as "stinking cedar", which he ascribes to the "strong and peculiar odour" of the timber, especially when it is "bruised or burnt". He also mentions the seed, covered in the aril, are approximately the size of a nutmeg.[17] In 1865 the German botanists Henkel and Hochstetter note that the Americans called the tree "stinking cedar" and "wild nutmeg". They mention that the name "nutmeg" is derived from the bone-hard shelled and acorn-sized seeds, which are covered in an aril somewhat similar to that of true nutmeg. They also describe that when the leaves are crushed they exude a pungent and disagreeable odour, which is why the local Americans used the name "stinking cedar". They themselves call the plant "Torrey's Nuss-Eibe", which translates as "Torrey's nut-yew" in English.[3] According to the British gardening writer Nelson in 1866, Torreya species in general were known as "stinking cedars" or "stinking nutmegs" by the locals, though he himself recommended the name "strong-odoured yew" as preferred for British use. This specific species was called "stinking cedar" by the Americans according to him, although he recommended the name "mountain yew" for Britain -this is a calque of the (incorrect) Latin name he was using, he was apparently unaware of the fact that this species grows almost at sea level and nowhere near any mountains![5] In fact, of all species of Torreya, it is the only species which is never found in mountainous areas in its habitat.
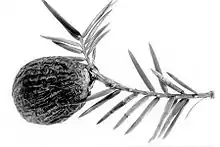
Although the vernacular name Florida torreya was formally recommended by most conservation works and internet databases,[6][7][9][28][8][26][24][29][25] this was changed to Florida nutmeg in the late 2010s,[7][30] after the IUCN invented a new name in 2010.[1] Other names for this tree are savin,[7][8][25] polecat wood,[7][25] yew-leaved torreya,[25][17] foetid yew,[25] stinking cedar,[1][31] mountain yew (Britain)[5] or stinking yew (applied to the Californian species Torreya californica).[5]
Description
Torreya taxifolia is an evergreen tree that may reach heights of 18 metres (59 ft) with an 80 centimetres (31 in) diameter trunk,[9] although it typically grew to 9–12 metres (30–39 ft) tall and 30–50 centimetres (12–20 in) in diameter,[7] and most stands today are composed of immature trees of less than 3 metres (9.8 ft) tall.[11] The crown is open and conical in overall shape,[9] with whorled branches.[28] These branches are spreading to slightly drooping. The bark of two-year-old branches are coloured yellowish-green, yellowish-brown or grey.[9] The young branchlets divide into threes.[17]
The stiff, needle-like leaves are sharp to the touch,[28] 1.5–3.8 centimetres (0.59–1.50 in) long[9] and 3 mm broad. These are arranged in two ranks on the branches[24] and are coloured glossy green above and light green below,[28] with a very slightly sunken grayish stripe of stomata on either side of the midrib on the underside,[9][28] and slightly round in transverse profile on the topside.[9] The leaves have an unpleasant, strongly pungent, resinous odor when crushed.[9][28]
It is dioecious, with separate male and female plants.[1] The male (pollen) cones resemble those of a common yew, but are much larger and have imbricated scales (bracts) at their base.[17] They are 5–7 mm long, grouped in lines along the underside of a shoot. The female (seed) cones are single or grouped two to five together on a short stem; minute at first, they mature in about 18 months to a drupe-like structure with the single large nut-like seed surrounded by a fleshy covering called an aril, 2.5–3.5 centimetres (0.98–1.38 in) long including aril,[9] about the size of a nutmeg.[17] The aril is glaucous and coloured dark green and streaked with purple at full maturity in the fall.[9] Unlike true yews, in which the aril forms a "cup" around the seed, in this plant the aril completely encloses the seed, leaving only a minute perforation at the end. The aril is fleshy in consistency, like a fruit.[17] When the aril is removed, the seed bears a striking resemblance to a large acorn.[3][17]
Distribution

Torreya taxifolia is restricted to limestone bluffs and ravines along the east bank of the Apalachicola River in the central part of the northern Florida Panhandle and immediately adjacent southernmost Georgia,[1] near the town of Chattahoochee, Florida;[25] there is also a small colony west of the Apalachicola at Dog Pond in Jackson County.[7][32] It grows along the Apalachicola River just south of the confluence of the Chattahoochee River and the Flint River, which reach northwards through Columbus and Atlanta to drain the southern Blue Ridge Mountains, a subrange of the Appalachian Mountains.[17] It only occurs in the Florida counties of Gadsden, Jackson and Liberty, and extends one mile into Decatur County, Georgia[7] (another source states the northernmost wild individual currently growing in Georgia is within 200 metres (660 ft) of the Florida state line). The outlying population west of the Apalachicola, in Jackson County, has always been small: In 1938 H. Kurz reported it to be restricted to one stand with a few trees,[33] in 1974 this was reported as consisting of 60 trees,[7] and in 1989 Mark William Schwartz and R. Nicholson reported it to consist of five trees.[33]
The area in which it naturally occurs is 203 square kilometres (50,000 acres), stretching 35 kilometres (22 mi) along the Apalachicola River.[33]
There is a small introduced population on the Biltmore Estate in Asheville, North Carolina, where it was planted as an ornamental plant.[7]
Prehistoric distribution
Though a number of sources state that this species has been recorded from fossils which suggest that at one time it grew over much of the eastern United States and that it has existed for 165 million years;[25][31] this is incorrect.[34] Only two fossil examples are known of Torreya in eastern North America. The first was a species with dense, spirally arranged leaves described as Tunion carolinianum by Edward W. Berry in 1908 from the Mid-Cretaceous of North Carolina,[35] the second is only known from a single piece of fossilised wood from the Upper Cretaceous, also from North Carolina, which has been described as Torreya antiqua.[36]
Ecology
It grows at altitudes of 15–30 metres (49–98 ft), mostly on wooded ravines, bluffs and steep,[1][9][8] north-facing slopes.[1] These ravines have nearly permanent seeps. It also occurs in the bottoms of ravines and adjacent floodplains.[8] It grows in the shade under the canopy of larger trees.[1][7][8] The soils of this region are calcareous,[8][17] moist, dark-coloured, sandy loams.[8]
It occurs in two types of habitats restricted to the river drainage, an oak-tupelo-cypress forest or an oak-pine forest.[7] These forests are mostly deciduous, but evergreen hardwoods and conifers are also common.[8] R. M. Harper, who travelled throughout northern Florida by horse and train to document the compositions of various forests, stated in 1914 that the most frequent species in the Apalachicola ravines were Magnolia grandiflora (9.5%), spruce pine (Pinus glabra, 5.6%, American beech (Fagus grandifolia, 4.1%) and the understory Torreya taxifolia (4.0%) and Ilex opaca (3.5%).[33] According to the IUCN the large trees in this habitat are Fagus grandifolia, tulip tree (Liriodendron tulipifera), Acer barbatum, sweetgum (Liquidambar styraciflua), white oak (Quercus alba), and occasionally loblolly pine (Pinus taeda) and Pinus glabra.[1] Magnolias also grow in these woods.[8] Often these woods are hung with vines such as Smilax species and crossvine (Bignonia capreolata).[1] Another rare conifer, the Florida yew (Taxus floridana), occasionally grows with Torreya taxifolia.[1][37] A rare salamander found in these ravines is Desmognathus apalachicolae. The ravines boast the southernmost parts of the ranges of a number of more northern species such as the plants Hydrangea quercifolia, Epigaea repens and Kalmia latifolia and the copperhead snake Agkistrodon contortrix.[37] Upland around these woodlands are flatwoods, which are a longleaf pine/wiregrass (Pinus palustris/Aristida stricta) sandhill plant community.[7][37] It is uncommon in its native habitats, with individuals spaced far apart.[7]
Various animals eat the seeds.[7] Rodents favour the seeds.[8] Squirrels apparently remove the seeds, which they eat, from the arils, which they do not eat, and often store them in caches for the winter, where they may sprout into new trees.[38]

In a 2001 newsletter, the writer Connie Barlow suggested that T. taxifolia may be an evolutionary anachronism similar to the Osage orange (Maclura pomifera) and Kentucky coffeetree (Gymnocladus dioicus), which are thought to have been dispersed by a now-extinct animal. She made this suggestion based on the fact that this species is rare, that it grew well in the cooler temperatures of North Carolina, and her belief that the species was only found on the east bank of the Apalachicola and was unable to disperse to the west bank with the animals she thought might be the main dispersal agents today, squirrels. She theorised than during the last interglacial (the geologically short periods between ice ages) and the proceeding ones, the species grew further north. According to her theories, because there might have been an unknown and now extinct animal in the past which was better suited to eat and defecate the seeds, or otherwise move the seeds, the species was now "stuck" behind the Apalachicola which served as a refuge habitat for a relict population during the long periods of glacial conditions which characterised the Pleistocene. She further supposed that because the arils contain terpenes which are usually toxic for mammals, and the seed's shell is so thin that mammal molars would crush the seeds, that the purported extinct animal might not be a mammal and instead suggested some unknown species of large tortoise as the extinct ecological partner.[38]
Uses
It has been far too rare to harvest commercially since the 1950s, but it has a beautiful, yellow-coloured, close-grained wood. The timber is lightweight, hard, strong and highly durable.[7] In the 19th century, the tree was harvested for wood that was used as fence posts,[7][8][26] shingles,[8][26] cabinets,[7] Christmas trees,[1][8][7] firewood,[8][26] and as a fuel for riverboats on the Apalachicola River.[1] Fences made of this wood in the 1910s were still good in the 1970s.[7] In the 1830s it was locally abundant enough for the trees to be harvested to be sawn into planks, which were much used in the construction of the village of Aspalaga Landing. In this era it was also recommended as making excellent posts for fencing, not being liable to attack by insects.[17]
When the trunks are damaged, the trees yield a small quantity of pasty, viscous, blood-red turpentine, which can be dissolved in alcohol, but has a very powerful and unpleasant odour.[17]
Cultivation
The tree is well-represented in cultivation, and is widely planted outside of its native range as an ornamental, and is found in private gardens, arboreta and botanical gardens.[8][26]
It was first imported to grow in Europe in 1840. Large trees were to be found in Germany by the 1860s.[3] It is tolerably hardy, but grows very slowly in Britain, and as such was only recommended for collectors.[5]
It has occasionally been planted as a landscape tree around Tallahassee, and one such specimen in Lee, Florida has achieved 30 feet (9.1 m).[8] Some large specimens are grown elsewhere in botanical gardens. The champion tree of the species is in a private garden in Norlina, North Carolina, having a height of 45 feet (14 m), a trunk diameter of 88 centimetres (35 in) dbh and a canopy width of 40 feet (12 m) in 1996.[8][26] It has been said to endure winter temperatures of −31 °C (−24 °F) in North Carolina without much problem.[26][38]
Propagation
Cuttings of Torreya shoot-tips often show fungal contamination. Experimental studies with weekly sprayed applications of a combinations of systemic fungicides, such as thiophanate-methyl combined with zinc and Maneb or thiabendazole, were able to eliminate the fungi from stock plants after four weeks, although these preparations were not US government approved as of 1987.[25]
Tissue culture methods of propagation were being investigated as of 1987.[25]
Conservation
The first person to notice that the trees were dying was the forester L. T. Nieland in 1938, although he never wrote a formal paper about it. In the same year Kurz made a detailed study of the ecology of species and mentioned that there was no danger to the species vanishing from its habitat if lumbering would cease.[24] In 1954 Kurz and R. K. Godfrey surveyed the population and noticed no symptoms of decline. However, in 1962 Godfrey and Kurz, having surveyed the species again, reported that the natural stands of this species, which was historically never widely distributed, were dying off and extinction seemed assured, if not already accomplished.[24][39] By 1962, only non-reproductive sprouts regrowing from the top-killed stumps remained in the wild,[26][25][39] a situation which has persisted throughout the 20th century.[9][8] They attributed this to deforestation due to the lumber trade.[24][39] However, samples of the afflicted trees were sent to the University of Florida, where Erdman West suggested the decline appeared due to a fungal blight of some kind. In a 1967 article S. A. Alfieri Jr. et al. of the Florida Department of Agriculture presented their research of the phenomenon. The disease presents itself as a small, yellowish spot on the needles (leaves) -one or more per leaf, which soon spreads throughout the leaf until it dies, turns brown and falls off. Subsequently, the disease spreads into the twig, and all of the needles fall off, leaving only a tuft of new growth at the tip. The disease spreads in stages, first affecting portions, later the entire tree. Severely infected trees eventually lose most of their needles and twigs. Fungal fruiting structures grow on wholly necrotic tissue on the underside of the leaves, twigs and buds. In some cases, the stems of affected or dead trees also showed fungal canker. Young seedlings are also affected, but less severely than older trees. Trees growing in the shade are more affected than those in full sunlight.[24]
In 1985 the phytopathologist Nabih Elias El-Gholl, also of the Florida Department of Agriculture, was able to prove that there were at least two pathogens involved; the leaf-spot disease presented as light greyish-green spots, which later became tan-coloured, and were up to 8.4mm long and as wide as the needle, with brown, irregularly-shaped, 2.4mm long × 2mm wide, necrotic centres and were able to infect the plants in the absence of wounds within three days of inoculation. Initially only a few spots, maximally four, developed per plant. After some two weeks the infected needles died and fell off -by this time the undersides had developed spore structures,[40] which were primarily found along the two grey bands of stomata, but may also occur on the twigs.[25]
The canker disease presents as elongated fusiform swellings on the stems which eventually may break open. They are often found at the base of the trees.[25]
In 1993 Schwartz, who has spent much of his career researching the conservation of this species, and Sharon M. Hermann, reported on the progression of the disease. They monitored a census population of some 100 trees for four years, in this time 10% died, mostly the smaller individuals. Most plants consisted of multi-stemmed, regenerating stumps; the mean length of the longest stems were less than one meter. Less than half of the plants showed any growth in length during these four years, and 32% lost their primary stem, which meant the mean size of individuals within the population was decreasing. Death was not strongly related to specific location or if the plants showed disease symptoms, although stem mortality was higher with trees showing a higher severity of foliar pathogens.[32] In 2000 Schwartz et al. reported that despite Godfrey and Kurz's 1962 prediction, a small population size, continued population losses, and no known seed production for decades, extinction of T. taxifolia had not yet occurred, and although the population would likely steadily decline, it was unlikely to go extinct within the next 50 years.[33]
Status
This was one of the first federally listed endangered plant species in the United States in 1984 (Federal Register 1/23/84).[9][25][10] It is one of only two species of conifer protected under the Endangered Species Act.[26] It was listed as "threatened" within the state of Florida by 1987 (Florida Statutes Section 581.185),[25] again in 1994 by the Florida Game and Fresh Water Fish Commission,[7] and in 1998 the status in Florida was changed to "endangered".[30][28] In 2002 it was added to the list of "endangered" plants of Georgia.[30][8] It has been a "critically endangered" species on the IUCN Red List since 1998, in 2000 this was said to be due to estimated 98% decline in mature individuals since the early 1950s.[1][11]
Population
In 2000 Schwartz and colleagues calculated that before the start of the decline, the original population of T. taxifolia may have been 300,000 between 650,000 plants, with 357,500 individual trees being the preferred estimate, based on a historic abundance of 14.2% of dominant ravine trees in 1914, or 30 trees/ha, and a historic distribution of some 203 square kilometres (50,000 acres).[33] Only 27 trees were counted in Georgia in 1981.[11] In 1993 Schwartz estimated that there were only 1500 trees in the wild.[8][32] Based on the extrapolation of a survey of five stands of the 2000 survey, attenuated with assumptions that these stands were the most dense, Schwartz et al. calculated the population in its native habitat to be between 500 and 4,000 individuals, with none capable of reproduction. The most likely population was calculated to be 1,361 individuals, not including the population west of the Apalachicola. Using their estimate of the pre-decline population, they thus calculated a minimum 98.5% decline in population over the last 50 years.[33] The IUCN estimated that the population is 999 mature individuals in its native habitat (although, confusingly, it also states the population to consist of between 500 and 600 trees elsewhere in the assessment), of which less than ten were known to produce male or female cones.[1] It stated that the population continued to decrease,[1] although the decline may be reversible should the causes of infection be better understood.[1][33] IUCN population viability analyses indicate that extinction within its native range is inevitable.[1]
The IUCN stated the total extent of occurrence is estimated to be about 200 square kilometres (49,000 acres),[1] this is the same as the historical distribution. Schwartz states that these has been no logging in its habitat since the 1950s and that this species has experienced no habitat loss: the vast majority of the original ravine forest habitat remains.[33]
Threats
The most significant current threat to the species is the continued reproductive failure associated with fungal pathogens.[1][29] Individuals do not reach reproductive size before being top-killed.[1] Almost no plants are able to reach a reproductive size, and where seeds do form, these are soft, crumbly and not viable.[7] Up to ten species of fungi have been found growing in infected Torreya taxifolia.[24][29][25][41]
Alfieri et al. were able to isolate five species of fungi in 1967 from the stems and leaves, but failed to isolate any of the fungi from the cankered stems on the media they were using, potato dextrose agar. They reported that the disease appeared to implicate species tentatively identified as Physalospora and Macrophoma, but also isolated the fungi Rhizoctonia solani, Sclerotium rolfsii and a Sphaeropsis species from the infected tissue.[24] They attempted to reproduce the infections by spraying the isolates, as well as pulverised stem and leaf litter, on seedlings grown in greenhouses from unaffected ornamental trees in local parks, but their experiments failed to induce the disease in their seedlings.[24] Nonetheless reports from 1975 and 1988 continued to identify the responsible fungi as these previous two species.[7] In 1985 El-Gholl was finally able to prove Koch's postulates with Gibberella baccata (as Fusarium lateritium, an anamorph isolate) as the agent responsible for the leaf spot pathogen, but he was unable to replicate the disease causing the stem cankers.[29][40][42] In 1987 Alfieri et al. reported on experiments with this fungus, as well as another two more species isolated from the infected plants, Xylocoremium flabelliforme (the anamorph state of Xylaria cubensis) and the Macrophoma which they had re-identified as a Phyllosticta species, the anamorph form of Guignardia. They were able to reproduce El-Gholl's results, but again failed to identify the canker producing agent, the other two isolates proved not to cause any disease.[29][25] In a 1996 article Lee et al. mentioned that the endophyte they were studying, Pestalotiopsis microspora might be the cause of the decline of the species, because a strain they isolated produced a hitherto unknown cytotoxin they named "torreyanic acid", a dimeric quinone.[43] P. microspora is a usually commensal fungus commonly found within the tissues of many plant species, and is only rarely a pathogen -in these cases it is an opportunistic pathogen.[29] However, recent research has identified a previously unknown species of Fusarium may be the cause.[1]
By 1987 the canker disease had spread to plants cultivated outside the native range such as in the University of Florida campus in Gainesville and in the Alfred B. Maclay Gardens State Park in Tallahassee.[25]
According to one older theory logging of shade trees may stress individuals of this species, which does not like being suddenly exposed to full sunlight, conducing infection.[7][24][29]
Another threat to the population is destruction by deer.[1] Deer preferentially select young trees of this species to rub their new antlers on, sometimes killing them. Feral pigs may also uproot and destroy seedlings.[7] Another possible cause of the historical decline may be changes in the environment due to fire suppression, and changes in water tables linked to the construction of dams.[1] Specifically, many trees were killed when the land was flooded[7] in the construction of the Jim Woodruff Dam impounding Lake Seminole.[31]
According to one writer, the population of the species may have been impacted by postglacial global warming, as it may possibly be better adapted to the cooler climate found in Florida during the last ice age. It may not have been able to move north due to poor dispersal abilities. This is based on the fact that the introduced population on the Biltmore Estate in North Carolina appears to be doing fine, and is even reproducing naturally -squirrels plant the seeds in the flower beds and these germinate.[38]
Actions
Fungicide treatment with Maneb has been shown to be remarkably effective, with plants showing renewed growth afterwards with little to no fungal infection. It has been applied since 1967,[7][24] but as of 1987 it is not specifically labelled for use in Torreya.[25]
A theory perhaps first put forward in 1990 by Greg Seamon, land manager at the Apalachicola Bluffs and Ravines Preserve, was that Torreya taxifolia may be somehow suffering from the suppression of fire in the upland longleaf pine-wiregrass sandhill ecosystems. However, after regular fires were again instituted in the 1990s in the preserve, this appeared not to have an effect, with the species experiencing an increased three percent annual mortality at the turn of the century.[37]
Two years after declaring the species an endangered species, in 1986, the U.S. Fish and Wildlife Service published a recovery plan. The chief tactic was to produce a genetically diverse collection of trees for reproduction and reintroduction into the wild. Other priorities were to protect the habitat of remaining populations, and to study the disease and methods of propagation.[8]
Plants in general attract very little funding compared to vertebrates, this tree is no different. The US federal government spent only $1,500 on conservation of the species in 1993, placing it 884th out of 926 endangered species. Schwartz et al. stated that recovery is possible, but this will require a greater level of commitment and a dedicated increase in funding.[33]
Small experiments by Schwartz and Hermann in the 1990s found that planting cuttings in the native habitat is possible. Schwartz et al. recommended population augmentation by planting volunteers isolated by more than 100m from extant stands to reduce chance of contamination.[33]
The tree is well-represented in cultivation, and is widely planted for conservation purposes outside of its native range.[8][26] Plants outside of its historic native distribution are undamaged by fungal disease and produce viable seed.[8][26][33] In Tallahassee for example, not far from the native range, a number of large trees had been grown for almost a century in the Alfred B. Maclay Gardens without problems and had been successfully propagated here since the 1960s,[24] although the trees in this park became infected by the canker disease by 1987.[25] It is found in arboreta, botanical gardens and elsewhere throughout the world.[8][26] In the Netherlands, for example, it is grown in the Arboretum Trompenburg, the Arboretum Oudenbosch, the Hortus Haren, the Hortus Botanicus Amsterdam[44] and the Pinetum Blijdenstein.[45]
In the United States the Center for Plant Conservation maintains the species in its National Collection of Endangered Plants; the Arnold Arboretum of Harvard University is the primary custodian of this species.[8] The Atlanta Botanical Garden and the State Botanical Garden of Georgia are actively propagating the plant for conservation purposes. These are members of the Georgia Plant Conservation Alliance, which is protecting the species by growing seedlings and cuttings, and planting these both in situ and ex situ.[8][31][46] Plants are also grown at the United States Botanic Garden.[31]
In her intriguing but somewhat unsubstantiated 2001 newsletter article Barlow synthesized her suppositions to suggest that the only way to conserve the species was for humans to engage in assisted migration, transplanting across great distances. She noted that, in essence, humans are now the gardeners artificially preserving most isolated islands of "wilderness" amongst a sea of human development, if we like it or not.[38] In this she was not unique, in 1994 Foote and Jones had already written that the survival of this species was likely dependent upon cultivation in gardens.[8] The idea is that T. taxifolia for some reason was unable to migrate north from its "ice age pocket refuge" in northern Florida.[47] This inspired the formation of a group, Torreya Guardians, which are attempting to rewild Torreya taxifolia trees in cooler mountain areas in the southern Appalachians in an attempt to aid the species.[48] Some conservationists consider T. taxifolia as the lead candidate for assisted migration.[47] This project has proved contentious.[49]
Protected areas
The natural populations are largely protected within the Torreya State Park[26][24] and at the Nature Conservancy's Apalachicola Bluffs and Ravines Preserve (first parcels purchased in 1982 to protect the ravines, before the species was officially listed as endangered).[37]
References and external links
| Wikimedia Commons has media related to Torreya taxifolia. |
- Spector, T.; Determann, R. & Gardner, M. (10 August 2010). "Torreya taxifolia". IUCN Red List of Threatened Species. 2011: e.T30968A9585489. doi:10.2305/IUCN.UK.2011-2.RLTS.T30968A9585489.en. Retrieved August 15, 2014.
Listed as Critically Endangered (CR A2ace v3.1)
- "Torreya taxifolia Arn". Plants of the World Online. Board of Trustees of the Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- Henkel, Johann Baptist; Hochstetter, Wilhelm Christian (1865). Synopsis der nadelhölzer, deren charakteristischen merkmale nebst andeutungen über ihre cultur und ausdauer in Deutschlands klima (in German). Stuttgart: Verlag der J. G. Cottaschen Buchhandlung. p. 367, 368. doi:10.5962/bhl.title.15349.
- "Taxus montana". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- Nelson, John (under the pseudonym Johannes Senilis) (1866). Pinaceae: being a handbook of the firs and pines. London: Hatchard and co. p. 167, 168. doi:10.5962/bhl.title.84872.
- Wunderlin, Richard P.; Hansen, Bruce F.; Franck, Alan R.; Essig, F. B. (17 March 2020). "Torreya taxifolia - Species Page". ISB Atlas of Florida Plants. Institute for Systematic Botany, University of South Florida, Tampa. Retrieved 18 March 2020.
- Esser, Lora L. (1993). Torreya taxifolia. In: Fire Effects Information System (Report). U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory. Retrieved 15 March 2020.
- Kadis, Irina (18 June 2008). "National Collection of Imperilled Plants (Center for Plant Conservation)". Archived from the original on 23 August 2009. Retrieved August 15, 2014.
- Hils, Matthew H. (1993). "Torreya taxifolia in Taxaceae Gray". Flora of North America, Vol. 2: Pteridophytes and Gymnosperms. New York and Oxford: Flora of North America Association. see also: RangeMap
- U.S. Fish and Wildlife Service. 1984. Federal Register (pdf)
- Conifer Specialist Group 2000 (2000). "Torreya taxifolia". IUCN Red List of Threatened Species. 2000: e.T30968A9585792. Retrieved 15 March 2020.
- McCormick, Carol Ann (4 January 2014). "Hardy Bryan Croom". Collectors of the UNC Herbarium. University of North Carolina Herbarium. Retrieved 17 March 2020.
- Flowers, John Baxton III (1979). "Croom, Hardy Bryan". Dictionary of North Carolina Biography. University of North Carolina Press. Retrieved 17 March 2020.
- Croom, John H. (19 December 2005). "Hardy Bryan Croom". Croom Families and Other Related Surnames. John H. Croom. Retrieved 17 March 2020.
- Conrad, Gibby (25 June 2012). "The life and death of Hardy Croom, first owner of Goodwood Plantation". Tallahassee Magazine. Tallahassee: Rowland Publishing. Retrieved 17 March 2020.
- Croom, Hardy Bryan (1834). Hardy Bryan Croom and John Torrey correspondence, 1834-1837. New York: New York Botanical Garden Archives.
- Nuttall, Thomas (1849). The North American sylva; or, A description of the forest trees of the United States, Canada and Nova Scotia. 3. Philadelphia: Smith & Wistar. p. 91, 92.
- "Torreya taxifolia". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- "Foetataxus taxifolia". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- "Tumion taxifolium". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- "Tumion". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- "Foetataxus montana". International Plant Names Index. Royal Botanic Gardens, Kew. 2020. Retrieved 16 March 2020.
- Christenhusz, J. M. M.; Reveal, J. L.; Martin, F. G.; Robert, R. M.; Chase, W. M. (2011). "Linear sequence, classification, synonymy, and bibliography of vascular plants: Lycophytes, ferns, gymnosperms and angiosperms" (PDF). Phytotaxa. 19: 1–134. doi:10.11646/phytotaxa.19.1.1. hdl:10138/28914.
- Alfieri, S. A. Jr.; Martinez, A. P.; Wehlburg, C. (1967). "Stem and needle blight of Florida Torreya, Torreya taxifolia Arn" (PDF). Proceedings of the Florida State Horticultural Society. 80: 428–431. S2CID 55845104. Retrieved 20 March 2020.
- Alfieri, S. A.; Schoulties, C. L.; Langdon, K. R.; El-Gholl, Nabih Elias (January 1987). Leaf and stem disease of Torreya taxifolia in Florida (PDF). Plant Pathology Circular (Report). Bureau of Plant Pathology, Florida Department Agriculture & Consumer Services. pp. 1–4. 291. Retrieved 22 March 2020.
- Earle, Christopher J. (17 January 2020). "Torreya taxifolia". The Gymnosperm Database. Christopher J. Earle. Retrieved 19 March 2020.
- Schütt, Peter; Weisgerber, Horst; Schuck, Hans J.; Lang, K. J.; Stimm, B.; Roloff, A. (2004). Lexikon der Nadelbäume (in German). Hamburg: Nikol-Verlagsges.mbH. ISBN 3-933203-80-5.
- Coile, Nancy C. (1998). Notes on Florida's endangered and threatened plants. Gainesville: Florida Department of Agriculture & Consumer Services, Division of Plant Industry. p. 55.
- Smith, Jason Andrew; Trulock, Aaron (November 2010). The Decline of Florida Torreya: An Endemic Conifer on the Edge of Extinction (Report). School of Forest Resources and Conservation, UF/IFAS Extension, University of Florida. FOR276. Retrieved 21 March 2020.
- "Torreya taxifolia". Natural Resources Conservation Service PLANTS Database. USDA. Retrieved 11 December 2015.
- "Stinking cedar". United States Botanic Garden. Retrieved 22 March 2020.
- Schwartz, Mark William; Hermann, Sharon M. (July 1993). "The Continuing Population Decline of Torreya taxifolia Arn". Bulletin of the Torrey Botanical Club. 120 (3): 275–286. doi:10.2307/2996992. JSTOR 2996992.
- Schwartz, Mark William; Hermann, Sharon M.; Van Mantgem, Philip J. (August 2000). "Estimating the magnitude of decline of the Florida torreya (Torreya taxifolia Arn.)". Biological Conservation. 95 (1): 77–84. doi:10.1016/S0006-3207(00)00008-2. Retrieved 19 March 2020.
- Barlow, Connie (August 2015). Paleoecology and the Assisted Migration Debate: Why a Deep-Time Perspective is Vital (Torreya taxifolia as exemplar) (Report). Torreya Guardians. p. 1—11. doi:10.13140/RG.2.1.4299.0166. Retrieved 22 March 2020.
- Berry, Edward Wilber (1 May 1908). "A Mid-Cretaceous species of Torreya; Tunion carolinianum sp. nov". American Journal of Science. 25 (149): 382–386. doi:10.2475/ajs.s4-25.149.382. Retrieved 22 March 2020.
- Boeshore, Irwin; Gray, William D. (1 October 1936). "An Upper Cretaceous Wood: Torreya antiqua". American Journal of Botany. 23 (8): 524–528. doi:10.1002/j.1537-2197.1936.tb09019.x.
- Vaughan, Elizabeth (2001). "Restoration of longleaf pine and wiregrass at the Apalachicola Bluffs Preserve (Florida)". Volume 7- Fall 2001: Lessons Learned From Long-Term Restorations. Restoration and Reclamation Review, Student On-Line Journal (Hort 5015/5071). University of Minnesota. Archived from the original on 2004-07-25. Retrieved 20 March 2020.
- Barlow, Connie (2001). "Anachronistic Fruits and the Ghosts Who Haunt Them" (PDF). Arnoldia. Vol. 61 no. 2. Boston, Massachusetts: Arnold Arboretum of Harvard University. pp. 19–21. Retrieved 2014-07-26.
- Godfrey, R. K.; Kurz, H. (1962). "The Florida Torreya destined for extinction". Science. 136 (3519): 900–902. Bibcode:1962Sci...136..900G. doi:10.1126/science.136.3519.900-a. PMID 17754185.
- El-Gholl, Nabih Elias (1985). "Fusarium lateritium Causing Needle Spots on Torreya taxifolia in Florida". Plant Disease. 69 (10): 905. doi:10.1094/PD-69-905a. Retrieved 21 March 2020.
- "Fusarium torreyae sp nov., a pathogen causing canker disease of Florida torreya (Torreya taxifolia), a critically endangered conifer restricted to northern Florida and southwestern Georgia". Retrieved 22 March 2020. Cite journal requires
|journal=(help) - Bensch, K. (2020). "Gibberella baccata". MycoBank. International Mycological Association (IMA) and the Westerdijk Fungal Biodiversity Institute. Retrieved 22 March 2020.
- Lee, Julie Chu-Li; Strobel, Gary A.; Lobkovsky, Emil; Clardy, Jon (17 May 1996). "Torreyanic Acid: A Selectively Cytotoxic Quinone Dimer from the Endophytic Fungus Pestalotiopsis microspora". The Journal of Organic Chemistry. 61 (10): 3232–3233. doi:10.1021/jo960471x.
- "Florida torreya". Plantzoeker (in Dutch). Nederlandse Vereniging van Botanische Tuinen. Retrieved 19 March 2020.
- Stichting Pinetum Blijdenstein (July 2016). Pinetum Blijdenstein — Beleidsplan 2016 – 2020 v.1.0 (Report) (in Dutch). Stichting Pinetum Blijdenstein. p. 22. Retrieved 19 March 2020.CS1 maint: uses authors parameter (link)
- GPCA Safeguarding Policy Statement - Policy Statement Regarding in situ and ex situ Plant Conservation Between Members of the Georgia Plant Conservation Alliance (PDF) (Report). Georgia Plant Conservation Alliance. April 2008. pp. 1–10. Retrieved 15 March 2020.
- Fox, Douglas (January 2007). "When worlds collide". Conservation. Vol. 8 no. 1. pp. 28–34.
- "Rewilding Torreya taxifolia?". Torreya Guardians. April 2015. Retrieved 22 March 2020.
- Nijhuis, Michelle (May–June 2008). "Taking Wildness in Hand: Rescuing Species". Orion Magazine. Great Barrington, Massachusetts: The Orion Society. pp. 64–78.

_(9150099237).jpg.webp)