Triphosgene
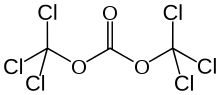
Triphosgene (bis(trichloromethyl) carbonate (BTC) is a chemical compound with the formula OC(OCCl3)2. It is used as a safer substitute for phosgene, because, at room temperature, it is a solid, whereas phosgene is a gas.[5] Triphosgene decomposes above 200 °C.[6]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bis(trichloromethyl) carbonate | |
| Other names
BTC | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.046.336 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3Cl6O3 | |
| Molar mass | 296.748 g/mol |
| Appearance | white solid |
| Density | 1.780 g/cm3 |
| Melting point | 80 °C (176 °F; 353 K) |
| Boiling point | 206 °C (403 °F; 479 K) |
| Reacts | |
| Solubility | *soluble in dichloromethane[1] |
| Hazards | |
| Safety data sheet | SDS Triphosgene |
| GHS pictograms |   [4] [4] |
| GHS Signal word | Danger |
| H314, H330[4] | |
| P260, P280, P284, P305+351+338, P310[4] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
This compound is commercially available. It is prepared by exhaustive free radical chlorination of dimethyl carbonate:[5]
- CH3OCO2CH3 + 6 Cl2 → CCl3OCO2CCl3 + 6 HCl
Triphosgene can be easily recrystallized from hot hexanes.
Uses
Triphosgene is used as a reagent in organic synthesis as a source of CO2+. It behaves like phosgene to which it cracks thermally:
- OC(OCCl3)2 → 3 OCCl2
Alcohols are converted to carbonates. Primary and secondary amines are converted to ureas and isocyanate.[5][7][8][9]
Safety
The toxicity of triphosgene and phosgene are the same since the trimer decomposes to phosgene on heating and upon reaction with nucleophiles. Trace moisture leads to formation of phosgene. Therefore, this reagent can be safely handled if one takes all the precautions as for phosgene.[10]
See also
References
- Michelle A. Ouimet, Nicholas D. Stebbins, Kathryn E. Uhrich (2013). "Biodegradable Coumaric Acid-Based Poly(anhydride-ester) Synthesis and Subsequent Controlled Release". Macromol. Rapid Commun. 34 (15): 1231–1236. doi:10.1002/marc.201300323. PMC 3789234. PMID 23836606.CS1 maint: multiple names: authors list (link)
- Tang, Shouwan; Ikai, Tomoyuki; Tsuji, Masashi; Okamoto, Yoshio (2010). "Immobilization and chiral recognition of 3,5-dimethylphenylcarbamates of cellulose and amylose bearing 4-(trimethoxysilyl)phenylcarbamate groups". Chirality. 22 (1): 165–172. doi:10.1002/chir.20722. PMID 19455617.
- Zhou, Yuhan; Gong, Runjun; Miao, Weirong (2006-09-01). "New Method of Synthesizing N‐Alkoxycarbonyl‐N‐arylamide with Triphosgene". Synthetic Communications. 36 (18): 2661–2666. doi:10.1080/00397910600764675. ISSN 0039-7911.
- Sigma-Aldrich Co., Triphosgene. Retrieved on 2018-06-12.
- Dr. Heiner Eckert; Dr. Barbara Forster (1987). "Triphosgene, a Crystalline Phosgene Substitute". Angew. Chem. Int. Ed. Engl. 26 (9): 894–895. doi:10.1002/anie.198708941.
- Dr. Heiner Eckert (2011). "Phosgenation Reactions with Phosgene from Triphosgene". Chim. Oggi Chem. Today. 29 (6): 40–46.
- Akiba, T.; Tamura, O.; Terashima, S. (1998). "(4R,5S)-4,5-Diphenyl-3-Vinyl-2-Oxazolidinone". Organic Syntheses. 75: 45. doi:10.15227/orgsyn.075.0045.
- Tsai, James H.; Takaoka, Leo R.; Powell, Noel A.; Nowick, James S. (2002). "Synthesis of Amino Acid Ester Isocyanates: Methyl (S)-2-Isocyanato-3-Phenylpropanoate". Organic Syntheses. 78: 220. doi:10.15227/orgsyn.078.0220.
- Du, Haifeng; Zhao, Baoguo; Shi, Yian (2009). "Pd(0)-Catalyzed Diamination of Trans-1-Phenyl-1,3-Butadiene with Di-tert-Butyldiaziridinone as Nitrogen Source". Organic Syntheses. 86: 315. doi:10.15227/orgsyn.086.0315.
- Suresh B. Damle (1993-02-08). "Safe handling of diphosgene, triphosgene". Chemical & Engineering News. 71 (6): 4.
