Tyrosol
Tyrosol is a phenylethanoid, a derivative of phenethyl alcohol. It is a natural phenolic antioxidant present in a variety of natural sources. The principal source in the human diet is olive oil. It is also one of the main natural phenols in argan oil. In olive oil, tyrosol forms esters with fatty acids.[1]
 | |
| Names | |
|---|---|
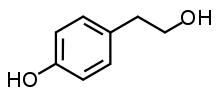
| IUPAC name
4-(2-Hydroxyethyl)phenol | |
| Other names
p-Hydroxyphenethyl alcohol 2-(4-Hydroxyphenyl)ethanol 4-Hydroxyphenylethanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.210 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H10O2 | |
| Molar mass | 138.164 g/mol |
| Melting point | 91 to 92 °C (196 to 198 °F; 364 to 365 K) |
| Boiling point | 158 °C (316 °F; 431 K) at 4 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Research
As an antioxidant, tyrosol can protect cells against injury due to oxidation in vitro.[2] Although it is not as potent as other antioxidants present in olive oil, its higher concentration and good bioavailability indicate that it may have an important overall effect.[3]
Tyrosol may also be cardioprotective. Samson et al. has shown that tyrosol-treated animals showed significant increase in the phosphorylation of Akt, eNOS and FOXO3a.[4] In addition, tyrosol also induced the expression of the protein SIRT1 in the heart after myocardial infarction in a rat MI model.
See also
References
- Lucas, Ricardo; Comelles, Francisco; AlcáNtara, David; Maldonado, Olivia S.; Curcuroze, Melanie; Parra, Jose L.; Morales, Juan C. (2010). "Surface-Active Properties of Lipophilic Antioxidants Tyrosol and Hydroxytyrosol Fatty Acid Esters: A Potential Explanation for the Nonlinear Hypothesis of the Antioxidant Activity in Oil-in-Water Emulsions". Journal of Agricultural and Food Chemistry. 58 (13): 8021–8026. doi:10.1021/jf1009928. PMID 20524658.
- Giovannini C, Straface E, Modesti D, Coni E, Cantafora A, De Vincenzi M, Malorni W, Masella R (1999). "Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells". J. Nutr. 129 (7): 1269–1277. doi:10.1093/jn/129.7.1269. PMID 10395586.
- Miró-Casas E, Covas M, Fitó M, Farré-Albadalejo M, Marrugat J, de la Torre R (2003). "Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans". European Journal of Clinical Nutrition. 57 (1): 186–190. doi:10.1038/sj.ejcn.1601532. PMID 12548315.
- Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N (2008). "Akt/FOXO3a/SIRT1-Mediated Cardioprotection by n-Tyrosol against Ischemic Stress in Rat in Vivo Model of Myocardial Infarction: Switching Gears toward Survival and Longevity". Journal of Agricultural and Food Chemistry. 56 (20): 9692–8. doi:10.1021/jf802050h. PMC 2648870. PMID 18826227.