Tocotrienol
The vitamin E family comprise four tocotrienols (alpha, beta, gamma, delta) and four tocopherols (alpha, beta, gamma, delta). The critical chemical structural difference between tocotrienols and tocopherols is that tocotrienols have unsaturated isoprenoid side chains with three carbon-carbon double bonds versus saturated side chains for tocopherols (see Figure).[1][2]
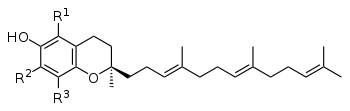
Tocotrienols are compounds naturally occurring at higher levels in some vegetable oils, including palm oil, rice bran oil, wheat germ, barley, saw palmetto, annatto, and certain other types of seeds, nuts and grains, and the oils derived from them.[3][4]
Chemically, different analogues of vitamin E all show some activity as a chemical antioxidant,[5] but do not all have the same vitamin E equivalence. Tocotrienols demonstrate activity depending on the type of antioxidant performance being measured.[6] All tocotrienols have some physical antioxidant activity due to an ability to donate a hydrogen atom (a proton plus electron) from the hydroxyl group on the chromanol ring, to free radical and reactive oxygen species. Historically studies of tocotrienols account for less than 1% of all research into vitamin E.[7] A scientific compilation of tocotrienol research, Tocotrienols: Vitamin E Beyond Tocopherols, was published in 2013.[3]
Health effects
A number of health benefits of tocotrienols have been proposed, included decreased risk of heart disease and cancer.[8] The Food and Nutrition Board of the Institute of Medicine of the United States National Academy of Sciences does not define a Recommended Dietary Allowance or Adequate Intake for tocotrienols.[9]
Brain
A review of human studies in middle-aged and elderly stated "Evidence from prospective and case-control studies suggested that increased blood levels of tocotrienols were associated with favorable cognitive function outcomes." The review qualified this statement by noting that randomized, controlled clinical trials were needed to evaluate these observations.[10]
Diabetes
Animal models and observational studies in humans have shown potential benefit.[8]
Cancer
Many studies have demonstrated anticancer potential of tocotrienols in vitro and in animal models. Two clinical studies of Phase I and Phase II now have shown that oral intake of tocotrienols created bioactive levels in the plasma and induced apoptosis in patients with pancreatic cancer.[12] In patients with ovarian cancer the combination of delta-tocotrienol with Bevacizumab was effective.[13] The researchers attribute the effects found to the anti-angiogenetic properties of tocotrienols, and their regulation of NF-κB.
Side effects
Tocotrienols are generally well tolerated and without significant side effects.[8]
History
The discovery of tocotrienols was first reported by Pennock and Whittle in 1964, describing the isolation of tocotrienols from rubber.[14] The biological significance of tocotrienols was clearly delineated in the early 1980s, when its ability to lower cholesterol was first reported by Qureshi and Elson in the Journal of Medicinal Chemistry.[15] During the 1990s, the anti-cancer properties of tocopherols and tocotrienols began to be delineated.[16] The current commercial sources of tocotrienol are rice and palm.[17] Other natural tocotrienol sources include rice bran oil, coconut oil, cocoa butter, barley, and wheat germ.[18] Tocotrienols are safe and human studies show no adverse effects with consumption of 240 mg/day for 48 months.[19] Tocotrienol rich fractions from rice, palm, or annatto, used in nutritional supplements, functional foods, and anti-aging cosmetics, are available in the market at 20%, 35%, 50%, and 70% total vitamin E content.
Etymology
Tocotrienols are named by analogy to tocopherols (from Greek words meaning to bear a pregnancy (see tocopherol); but with this word changed to include the chemical difference that tocotrienols are trienes, meaning that they share identical structure with the tocopherols except for the addition of the three double bonds to their side chains.
Comparison to tocopherols
Tocotrienols have only a single chiral center—the 2' carbon on the chromanol ring, which is where the isoprenoid tail is attached. Unlike the tocopherols, which have additional chiral centers along their saturated tail chain, the unsaturated chain of the tocotrienols instead have double-bonds at this sites. Tocotrienols extracted from plants are always dextrorotatory stereoisomers, signified as d-tocotrienols. In theory, (levorotatory; l-tocotrienol) forms of tocotrienols could exist as well, which would have a 2S rather than 2R configuration at the molecules' single chiral center, but unlike synthetic, dl-alpha-tocopherol, the marketed tocotrienol dietary supplements are all d-tocotrienol extracts from palm or annatto oils.
Tocotrienol studies confirm anti-oxidation,[20] anti-inflammatory potentials and suggest anti-cancer effects[21][22] better than the common forms of tocopherol due to their chemical structure. Scientists have suggested tocotrienols are better antioxidants than tocopherols.[23][24][25][26] It has been proposed that the unsaturated side-chain in tocotrienols causes them to penetrate tissues with saturated fatty layers more efficiently than tocopherol.[27] Lipid ORAC values are highest for δ-tocotrienol.[28] However that study also says: "Regarding α-tocopherol equivalent antioxidant capacity, no significant differences in the antioxidant activity of all vitamin E isoforms were found."
Metabolism and bioavailability
The metabolism and thus the bioavailability of tocotrienols are not well understood and simply increasing the intake of tocotrienols might not increase tocotrienol levels in the body.[29]
α-Tocopherol interference
Various studies have shown that alpha-tocopherol interferes with tocotrienol benefits.[29] High levels of α-tocopherol increase cholesterol production.[30] α-Tocopherol interference with tocotrienol absorption was described previously by scientists, who showed that α-tococopherol interfered with absorption of α-tocotrienol, but not γ-tocotrienol.[31] Finally, α-tocopherol was shown to interfere with tocotrienols by increasing catabolism.[32]
Sources
In nature, tocotrienols are present in many plants and fruits. The palm fruit (Elaeis guineensis) is particularly high in tocotrienols, primarily gamma-tocotrienol, alpha-tocotrienol and delta-tocotrienol. Other cultivated plants high in tocotrienols includes rice, wheat, barley, rye and oat.[33] In annatto, tocotrienols are relatively abundant (only delta- and gamma-tocotrienol however) and it contains no tocopherols.[34]
Research
Radiation countermeasures
No human trials. Following exposure to gamma radiation, hematopoietic stem cells (HSCs) in the bone marrow, which are important for producing blood cells, rapidly undergo apoptosis (cell death). There are no known treatments for this acute effect of radiation.[35] Two studies conducted by the U.S. Armed Forces Radiobiology Research Institute (AFRRI) found that treatment with γ-tocotrienol or δ-tocotrienol enhanced survival of hematopoietic stem cells, which are essential for renewing the body's supply of blood cells.[35][36] Based on these successful results of studies in mice, γ-tocotrienol is being studied for its safety and efficacy as a radioprotective measure in nonhuman primates.[37]
References
- Kamal-Eldin A, Appelqvist LA (July 1996). "The chemistry and antioxidant properties of tocopherols and tocotrienols". Lipids. 31 (7): 671–701. doi:10.1007/BF02522884. PMID 8827691.
- Clarke MW, Burnett JR, Croft KD (2008). "Vitamin E in human health and disease". Critical Reviews in Clinical Laboratory Sciences. 45 (5): 417–50. doi:10.1080/10408360802118625. PMID 18712629.
- Tan B, Watson RR, Preedy VR, eds. (2013). Tocotrienols: Vitamin E Beyond Tocopherols (2nd ed.). Boca Raton: CRC Press. ISBN 9781439884416.
- Sen CK, Rink C, Khanna S (June 2010). "Palm oil-derived natural vitamin E alpha-tocotrienol in brain health and disease". Journal of the American College of Nutrition. 29 (3 Suppl): 314S–323S. doi:10.1080/07315724.2010.10719846. PMC 3065441. PMID 20823491.
- Cerecetto H, López GV (March 2007). "Antioxidants derived from vitamin E: an overview". Mini Reviews in Medicinal Chemistry. 7 (3): 315–38. doi:10.2174/138955707780059871. PMID 17346221.
- Fu JY, Che HL, Tan DM, Teng KT (January 2014). "Bioavailability of tocotrienols: evidence in human studies". Nutrition & Metabolism. 11 (1): 5. doi:10.1186/1743-7075-11-5. PMC 3895660. PMID 24410975.
- Sen CK, Khanna S, Roy S (2007). "Tocotrienols in health and disease: the other half of the natural vitamin E family". Molecular Aspects of Medicine. 28 (5–6): 692–728. doi:10.1016/j.mam.2007.03.001. PMC 2435257. PMID 17507086.
- Meganathan P, Fu JY (October 2016). "Biological Properties of Tocotrienols: Evidence in Human Studies". International Journal of Molecular Sciences. 17 (11): 1682. doi:10.3390/ijms17111682. PMC 5133770. PMID 27792171.
- Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals (Report). Food and Nutrition Board, Institute of Medicine, National Academies. 2004. Archived from the original on 2010-05-24. Retrieved 2009-06-09 – via www.iom.edu.
- Georgousopoulou EN, Panagiotakos DB, Mellor DD, Naumovski N (January 2017). "Tocotrienols, health and ageing: A systematic review" (PDF). Maturitas. 95: 55–60. doi:10.1016/j.maturitas.2016.11.003. PMID 27889054.
- Prasad K (2011). "Tocotrienols and cardiovascular health". Current Pharmaceutical Design. 17 (21): 2147–54. doi:10.2174/138161211796957418. PMID 21774782.
- Springett, Gregory M.; Husain, Kazim; Neuger, Anthony; Centeno, Barbara; Chen, Dung-Tsa; Hutchinson, Tai Z.; Lush, Richard M.; Sebti, Saïd; Malafa, Mokenge P. (2015). "A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia". EBioMedicine. 2 (12): 1987–1995. doi:10.1016/j.ebiom.2015.11.025. ISSN 2352-3964.
- Thomsen, Caroline Brenner; Andersen, Rikke Fredslund; Steffensen, Karina Dahl; Adimi, Parvin; Jakobsen, Anders (2019). "Delta tocotrienol in recurrent ovarian cancer. A phase II trial". Pharmacological Research. 141: 392–396. doi:10.1016/j.phrs.2019.01.017. ISSN 1043-6618.
- Dunphy, P. J.; Whittle, K. J.; Pennock, J. F.; Morton, R. A. (1965). "Identification and Estimation of Tocotrienols in Hevea Latex". Nature. 207 (4996): 521–522. doi:10.1038/207521a0.
- Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ (October 1992). "Hypocholesterolemic activity of synthetic and natural tocotrienols". Journal of Medicinal Chemistry. 35 (20): 3595–606. doi:10.1021/jm00098a002. PMID 1433170.
- Watson & Preedy 2008, p. 6
- Tan, B. and M.H. Saleh, Integrated process for recovery of carotenoids and tocotrienols from oil in USPTO 5,157,132. 1992
- Packer L, Weber SU, Rimbach G (February 2001). "Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling". The Journal of Nutrition. 131 (2): 369S–73S. doi:10.1093/jn/131.2.369S. PMID 11160563.
- Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML (December 1995). "Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis". Lipids. 30 (12): 1179–83. doi:10.1007/BF02536621. PMID 8614310.
- Serbinova E, Kagan V, Han D, Packer L (1991). "Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol". Free Radical Biology & Medicine. 10 (5): 263–75. doi:10.1016/0891-5849(91)90033-Y. PMID 1649783.
- Constantinou C, Papas A, Constantinou AI (August 2008). "Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs". International Journal of Cancer. 123 (4): 739–52. doi:10.1002/ijc.23689. PMID 18512238.
- Wada S (2009). "Chemoprevention of tocotrienols: the mechanism of antiproliferative effects". Food Factors for Health Promotion. Forum of Nutrition. 61. pp. 204–16. doi:10.1159/000212752. ISBN 978-3-8055-9097-6. PMID 19367124.
- Müller L, Theile K, Böhm V (May 2010). "In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma". Molecular Nutrition & Food Research. 54 (5): 731–42. doi:10.1002/mnfr.200900399. PMID 20333724.
- Yoshida Y, Niki E, Noguchi N (March 2003). "Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects". Chemistry and Physics of Lipids. 123 (1): 63–75. doi:10.1016/S0009-3084(02)00164-0. PMID 12637165.
- Schaffer S, Müller WE, Eckert GP (February 2005). "Tocotrienols: constitutional effects in aging and disease". The Journal of Nutrition. 135 (2): 151–4. doi:10.1093/jn/135.2.151. PMID 15671205.
- Theriault A, Chao JT, Wang Q, Gapor A, Adeli K (July 1999). "Tocotrienol: a review of its therapeutic potential". Clinical Biochemistry. 32 (5): 309–19. doi:10.1016/S0009-9120(99)00027-2. PMID 10480444.
- Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L (October 1993). "Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency". Biochemistry. 32 (40): 10692–9. doi:10.1021/bi00091a020. PMID 8399214.
- Müller L, Theile K, Böhm V (May 2010). "In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma". Molecular Nutrition & Food Research. 54 (5): 731–42. doi:10.1002/mnfr.200900399. PMID 20333724.
- Fu JY, Che HL, Tan DM, Teng KT (January 2014). "Bioavailability of tocotrienols: evidence in human studies". Nutrition & Metabolism. 11 (1): 5. doi:10.1186/1743-7075-11-5. PMC 3895660. PMID 24410975.
- Stocker A (December 2004). "Molecular mechanisms of vitamin E transport". Annals of the New York Academy of Sciences. 1031: 44–59. doi:10.1196/annals.1331.005. PMID 15753133.
- Ikeda S, Tohyama T, Yoshimura H, Hamamura K, Abe K, Yamashita K (February 2003). "Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats". The Journal of Nutrition. 133 (2): 428–34. doi:10.1093/jn/133.2.428. PMID 12566479.
- Sontag TJ, Parker RS (May 2007). "Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase". Journal of Lipid Research. 48 (5): 1090–8. doi:10.1194/jlr.M600514-JLR200. PMID 17284776.
- Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet p.199
- Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry
- Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, et al. (December 2010). "Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling". Haematologica. 95 (12): 1996–2004. doi:10.3324/haematol.2010.026492. PMC 2995556. PMID 20823133.
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, et al. (June 2010). "Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation". Radiation Research. 173 (6): 738–47. doi:10.1667/RR1824.1. PMID 20518653.
- Singh VK, Beattie LA, Seed TM (November 2013). "Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures". Journal of Radiation Research. 54 (6): 973–88. doi:10.1093/jrr/rrt048. PMC 3823775. PMID 23658414.
External links
- Vitamin E factsheet — Office of Dietary Supplements, National Institutes of Health
- Tocotrienols at the US National Library of Medicine Medical Subject Headings (MeSH)
- Watson RR, Preedy VR, eds. (2008). Tocotrienols: Vitamin E beyond Tocopherols. Boca Raton: CRC Press. ISBN 978-1-4200-8037-7.