Vanadium oxydichloride
Vanadium oxydichloride is the inorganic compound with the formula VOCl2. One of several oxychlorides of vanadium, it is a hygroscopic green solid. It is prepared by comproportionation of vanadium trichloride and vanadium(V) oxides:[1]
- V2O5 + VOCl3 + 3 VCl3 → 6 VOCl2
 | |
| Names | |
|---|---|
| Other names
Vanadyl dichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.457 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cl2OV | |
| Molar mass | 137.84 g·mol−1 |
| Appearance | Green solid |
| Density | 2.88 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
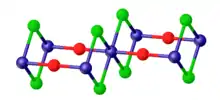
As verified by X-ray crystallography, vanadium oxydichloride adopts a layered structure, featuring octahedral vanadium centers linked by doubly bridging oxide and chloride ligands.[2]
References
- G. Brauer (1963). "Vanadium Oxydichloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1263.
- Seifert, H. J.; Uebach, J. (1981). "Beitrage zur Chemie und Struktur von Vanadylhalogeniden". Zeitschrift für anorganische und allgemeine Chemie. 479: 32–40. doi:10.1002/zaac.19814790804.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.