Virulent Newcastle disease
Virulent Newcastle disease (VND), formerly exotic Newcastle disease,[1] is a contagious viral avian disease affecting many domestic and wild bird species; it is transmissible to humans.[2] Though it can infect humans, most cases are non-symptomatic; rarely it can cause a mild fever and/or conjunctivitis in humans. Its effects are most notable in domestic poultry due to their high susceptibility and the potential for severe impacts of an epizootic on the poultry industries. It is endemic to many countries.
| Avian orthoavulavirus 1 | |
|---|---|
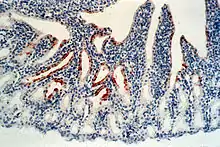 | |
| Avian orthoavulavirus 1 (stained in brown) in the conjunctiva of a chicken | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Paramyxoviridae |
| Genus: | Orthoavulavirus |
| Species: | Avian orthoavulavirus 1 |
Exposure of humans to infected birds (for example in poultry processing plants) can cause mild conjunctivitis and influenza-like symptoms, but the Newcastle disease virus (NDV) otherwise poses no hazard to human health. No treatment for NDV is known, but the use of prophylactic vaccines[3] and sanitary measures reduces the likelihood of outbreaks.
History
Newcastle disease was first identified in Java, Indonesia, in 1926, and in Newcastle-upon-Tyne, England, in 1927. However, it may have been prevalent as early as 1898, when a disease wiped out all the domestic fowl in northwest Scotland.[4]
The policy of slaughter ceased in England and Wales on 31 March 1963, except for the peracute form of Newcastle disease and for fowl plague. In Scotland the slaughter policy continued for all types of fowl pest.[5]
Interest in the use of NDV as an anticancer agent has arisen from the ability of NDV to selectively kill human tumour cells with limited toxicity to normal cells.
Since May 2018, California Department of Food and Agriculture staff and the United States Department of Agriculture have been working on eliminating VND in South California and more than 400 birds have been confirmed to have VND.[6][7] On February 27, 2019, California State Veterinarian, Dr. Annette Jones, increased the quarantine area in Southern California and on March 15, 2019 and April 5, 2019, cases of VND in Northern California and Arizona respectively.[8]
Causal agent
The causal agent, Newcastle disease virus (NDV), is a variant of avian orthoavulavirus 1, a negative-sense, single-stranded RNA virus. NDV belongs to the subfamily Avulavirinae, which infect birds. Transmission occurs by exposure to faecal and other excretions from infected birds, and through contact with contaminated food, water, equipment, and clothing.
Strains
NDV strains can be categorised as velogenic (highly virulent), mesogenic (intermediate virulence), or lentogenic (nonvirulent). Velogenic strains produce severe nervous and respiratory signs, spread rapidly, and cause up to 90% mortality. Mesogenic strains cause coughing, affect egg quality and production, and result in up to 10% mortality. Lentogenic strains produce mild signs with negligible mortality.
Use as an anticancer agent
In 1999, promising results were reported using an attenuated strain of the Newcastle virus, code named MTH-68, in cancer patients[9] by researchers who had isolated the strain in 1968.[10][11] It appears the virus preferentially targets and replicates in certain types of tumor cells, leaving normal cells almost unaffected. In 2006, researchers from the Hebrew University also succeeded in isolating a variant of the NDV, code named NDV-HUJ, which showed promising results in 14 glioblastoma multiforme patients.[12] In 2011, researchers at the Memorial Sloan–Kettering Cancer Center and the Icahn School of Medicine at Mount Sinai found that NDV modified with the viral protein NS1 had enhanced replication in cancer cell lines overexpressing the antiapoptotic factor Bcl-xL. The researchers suggested in cells that resist the normal inducement of apoptosis when infected will give NDV more time to incubate in cell and spread. Many cancer cells will overexpress antiapoptotic factors as part of tumor development. This mechanism of delaying apoptosis in abnormal cells gives NDV the specificity it needs to be an efficient cancer-fighting oncolytic virus.[13]
History of NDV in cancer therapy
Though the oncolytic effect of NDV was documented already in the 1950s, the main advances of viruses in cancer therapy came with the advent of reverse genetics technologies [14][15] With these new possibilities, studies of modified NDV strains with enhanced cancer-treatment properties have been put on the agenda. A study[15] demonstrated the engineered Hitcher B1 NDV/F3aa strain could be modified to express a highly fusogenic F-protein in combination with immunostimulatory molecules such as IFN-gamma, interleukin 2, or tumor necrosis factor alpha. Promising results were discovered with proteins associated to the adaptive immune system, which paved the way for possibilities to use NDV to create a tumor-associated antigen. Another study showed how NDV/F3aa could be modified to express NS1, an influenza virus protein with capability to modulate with the innate immune response, for example, by suppressing the induction of the cellular interferons.[16]
NDV pros and cons in cancer therapy
NDV possesses many unique anticancer properties and thereby provides an excellent base in virotherapy research. NDV has selectivity on oncogenic cells, where it replicates without, or in a less pronounced way, harming normal cells.[17][18] It binds, fuses into and replicates within the infected cells’ cytoplasm independent of cell proliferation.[17] One of the main issues using NDV treatment is the host/patient immune response against the virus itself, which prior to the time of the reverse genetics technology, decreased the applicability of NDV as a cancer treatment.[14][19]
NDV-induced mechanisms leading to tumor cell death
The precise way in which the presence of NDV induces tumor cell death remains to be clarified and may show variation regarding the strains of NDV used and which type of cancer is targeted. NDV triggers apoptosis[17] in a wide range of cancer cell types via the mitochondrial/intrinsic pathway, through loss of membrane potential and thereby inducing release of cytochrome c in the tumor cell. The results[17] also indicate the extrinsic pathway is activated by TNF-related, apoptosis-inducing ligand-induced, NDV-mediated apoptosis in a late stage. Another study[20] found a hyperfusogenic NDV/F3aa(L289A) with refined abilities to fuse into somatic cells. NDV has aggregating properties causing syncytia formations of tumor cells, which, apart from amplifying immune-based cell killing, also results in necrosis of cells. This pathway was believed to lead to a considerable boost of immune activation and potentially an antitumor response, which was supported by observations of a significant accumulation of NK-cells and neutrophils following the infusion of NDV/F3aa(L289A) in hepatocellular carcinoma cells. In addition, an increase of CD4+ and CD8+ T-cells occurs within the tumor cells when inducing NDV/F3aa recombined with the cytokine interleukin-2 (IL-2).[21] An NDV/F3aa-IL-2 strain induced the immune system, giving a cytotoxic effect on the tumor cells.[22] A 15-year study on patients with malignant melanoma showed increased numbers of oligoclonal CD8+ T-cells in the blood, suggesting vaccination with NDV oncolysates was associated with prolonged survival among the patients, and CD8+ T-cells played an important role.![23]
Transmission
NDV is spread primarily through direct contact between healthy birds and the bodily discharges of infected birds. The disease is transmitted through infected birds' droppings and secretions from the nose, mouth, and eyes. NDV spreads rapidly among birds kept in confinement, such as commercially raised chickens.
High concentrations of the NDV are found in birds' bodily discharges; therefore, the disease can be spread easily by mechanical means. Virus-bearing material can be picked up on shoes and clothing and carried from an infected flock to a healthy one.
NDV can survive for several weeks in a warm and humid environment on birds' feathers, manure, and other materials. It can survive indefinitely in frozen material. However, the virus is destroyed rapidly by dehydration and by the ultraviolet rays in sunlight. Smuggled pet birds, especially Amazon parrots from Latin America, pose a great risk of introducing NDV into the US. Amazon parrots are carriers of the disease, but do not show symptoms, and are capable of shedding NDV for more than 400 days.
Clinical findings
Clinical signs
Signs of infection with NDV vary greatly depending on factors such as the strain of virus and the health, age and species of the host.
The incubation period for the disease ranges from 4 to 6 days. An infected bird may exhibit several signs, including respiratory signs (gasping, coughing), nervous signs (depression, inappetence, muscular tremors, drooping wings, twisting of head and neck, circling, complete paralysis), swelling of the tissues around the eyes and neck, greenish, watery diarrhea, misshapen, rough- or thin-shelled eggs and reduced egg production.
In acute cases, the death is very sudden, and, in the beginning of the outbreak, the remaining birds do not seem to be sick. In flocks with good immunity, however, the signs (respiratory and digestive) are mild and progressive, and are followed after 7 days by nervous symptoms, especially twisted heads.
 Torticollis in a mallard
Torticollis in a mallard Same symptom in a broiler
Same symptom in a broiler PM lesions on proventriculus, gizzard, and duodenum
PM lesions on proventriculus, gizzard, and duodenum
Postmortem lesions
Petechiae in the proventriculus and on the submucosae of the gizzard are typical; also, severe enteritis of the duodenum occurs. The lesions are scarce in hyperacute cases (first day of outbreak).
Diagnosis
Immunological tests
Enzyme-linked immunosorbent assay, polymerase chain reaction, and sequence technology tests have been developed.
Virus isolation
Samples
For routine isolation of NDV from chickens, turkeys, and other birds, samples are obtained by swabbing the trachea and the cloaca. Cotton swabs can be used. The virus can also be isolated from the lungs, brain, spleen, liver, and kidneys.
Handling
Prior to shipping, samples should be stored at 4 °C (refrigerator). Samples must be shipped in a padded envelope or box. Samples may be sent by regular mail, but overnight is recommended.[24]
Prevention
Any animals showing symptoms of Newcastle disease should be isolated immediately. New birds should also be vaccinated before being introduced to a flock. An inactivated viral vaccine is available, as well as various combination vaccines.[3][25][26] A thermotolerant vaccine is available for controlling Newcastle disease in underdeveloped countries.[27]
References
- "Virulent Newcastle Disease (vND)". United States Department of Agriculture. Retrieved 6 April 2019.
- Nelson, CB; Pomeroy, BS; Schrall, K; Park, WE; Lindeman, RJ (Jun 1952). "An outbreak of conjunctivitis due to Newcastle disease virus (NDV) occurring in poultry workers". American Journal of Public Health and the Nation's Health. 42 (6): 672–8. doi:10.2105/ajph.42.6.672. PMC 1526237. PMID 14924001.
- FAO Manual on Vaccines
- Macpherson, LW (May 1956). "Some Observations On The Epizootiology Of NewCastle Disease". Canadian Journal of Comparative Medicine and Veterinary Science. 20 (5): 155–68. PMC 1614269. PMID 17648892.
- "Newcastle disease: Newcastle disease outbreaks in Great Britain". DEFRA. Archived from the original on 2007-06-27.
- "California modifies virulent Newcastle disease quarantine boundaries". Feedstuffs. 27 February 2019. Retrieved 6 April 2019.
- "USDA confirms virulent Newcastle disease in Arizona". Feedstuffs. 5 April 2019. Retrieved 6 April 2019.
- "Virulent Newcastle Disease". California Department of Food and Agriculture. Retrieved 6 April 2019.
- Csatary, LK; Moss, RW; Beuth, J; Töröcsik, B; Szeberenyi, J; Bakacs, T (Jan–Feb 1999). "Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H)". Anticancer Research. 19 (1B): 635–8. PMID 10216468.
- Csatary, LK; Csatary, E; Moss, RW (Mar 15, 2000). "Re: Scientific interest in Newcastle disease virus is reviving". Journal of the National Cancer Institute. 92 (6): 493–4. doi:10.1093/jnci/92.6.493. PMID 10716968.
- Csatary, LK (October 1971). "Viruses in the treatment of cancer". Lancet. 2 (7728): 825. doi:10.1016/s0140-6736(71)92788-7. PMID 4106650.
- Viruses: The new cancer hunters. isracast.com. 1 March 2006
- Mansour, M.; Palese, P.; Zamarin, D. (2011). "Oncolytic Specificity of Newcastle Disease Virus Is Mediated by Selectivity for Apoptosis-Resistant Cells". Journal of Virology. 85 (12): 6013–23. doi:10.1128/JVI.01537-10. PMC 3126310. PMID 21471241.
- Mullen, JT; Tanabe, KK (2002). "Viral oncolysis". The Oncologist. 7 (2): 106–19. doi:10.1634/theoncologist.7-2-106. PMID 11961194.(Flanagan et al. 1955 in Mullen & Tanabe 2002
- Vigil, A; Park, MS; Martinez, O; Chua, MA; Xiao, S; Cros, JF; Martínez-Sobrido, L; Woo, SL; García-Sastre, A (Sep 1, 2007). "Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus". Cancer Research. 67 (17): 8285–92. doi:10.1158/0008-5472.CAN-07-1025. PMID 17804743.
- Zamarin, D; Martínez-Sobrido, L; Kelly, K; Mansour, M; Sheng, G; Vigil, A; García-Sastre, A; Palese, P; Fong, Y (Apr 2009). "Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses". Molecular Therapy. 17 (4): 697–706. doi:10.1038/mt.2008.286. PMC 2835121. PMID 19209145.
- Elankumaran, S; Rockemann, D; Samal, SK (Aug 2006). "Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death". Journal of Virology. 80 (15): 7522–34. doi:10.1128/JVI.00241-06. PMC 1563725. PMID 16840332.
- Fábián, Z; Csatary, CM; Szeberényi, J; Csatary, LK (Mar 2007). "p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines". Journal of Virology. 81 (6): 2817–30. doi:10.1128/JVI.02490-06. PMC 1865991. PMID 17215292.
- Kuruppu, D; Tanabe, KK (May 2005). "Viral oncolysis by herpes simplex virus and other viruses". Cancer Biology & Therapy. 4 (5): 524–31. doi:10.4161/cbt.4.5.1820. PMID 15917655.
- Altomonte, J; Marozin, S; Schmid, RM; Ebert, O (Feb 2010). "Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma". Molecular Therapy. 18 (2): 275–84. doi:10.1038/mt.2009.231. PMC 2839313. PMID 19809404.
- Vigil, A; Martinez, O; Chua, MA; García-Sastre, A (Nov 2008). "Recombinant Newcastle disease virus as a vaccine vector for cancer therapy". Molecular Therapy. 16 (11): 1883–90. doi:10.1038/mt.2008.181. PMC 2878970. PMID 18714310.
- Zamarin, D; Vigil, A; Kelly, K; García-Sastre, A; Fong, Y (Jun 2009). "Genetically engineered Newcastle disease virus for malignant melanoma therapy". Gene Therapy. 16 (6): 796–804. doi:10.1038/gt.2009.14. PMC 2882235. PMID 19242529.
- Batliwalla, FM; Bateman, BA; Serrano, D; Murray, D; Macphail, S; Maino, VC; Ansel, JC; Gregersen, PK; Armstrong, CA (Dec 1998). "A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire". Molecular Medicine (Cambridge, Massachusetts). 4 (12): 783–94. PMC 2230393. PMID 9990864.
- Newcastle Disease Virus (NDV) Archived 2017-11-01 at the Wayback Machine. avianbiotech.com
- Newcastle Disease Vaccine, B1 Type, B1 Strain, Live Virus. drugs.com
- Merck/Intervet Vaccine
- Robyn Alders; Spradbrow, Peter (2001). Controlling Newcastle disease in village chickens : a field manual. Canberra: ACIAR. ISBN 978-1863203074.
External links
| Wikimedia Commons has media related to Newcastle disease virus. |
- Current status of Newcastle disease worldwide at OIE. WAHID Interface - OIE World Animal Health Information Database
- Disease card
- Department of Environment, Food and Rural Affairs, UK
- Newcastle Disease, Iowa State University, Center for Food Security and Public Health
- Newcastle Disease in Poultry, Merck Veterinary Manual
- Species Profile—Virulent Newcastle Disease, National Invasive Species Information Center, United States National Agricultural Library. Lists general information and resources for Exotic Newcastle Disease.