Whewellite
Whewellite /ˈhjuːəlaɪt/ is a mineral, hydrated calcium oxalate, formula Ca C2O4·H2O. Because of its organic content it is thought to have an indirect biological origin; this hypothesis is supported by its presence in coal and sedimentary nodules. However, it has also been found in hydrothermal deposits where a biological source appears improbable. For this reason, it may be classed as a true mineral.
| Whewellite | |
|---|---|
 A white Whewellite crystal from Schlema, Germany | |
| General | |
| Category | Oxalate minerals |
| Formula (repeating unit) | CaC2O4·H2O |
| Strunz classification | 10.AB.45 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | P21/c (no. 14) |
| Identification | |
| Color | Colorless, yellowish, brownish |
| Crystal habit | Equant or stout prismatic crystals |
| Twinning | e {101} twin plane |
| Cleavage | {101} good, {010} imperfect, {001} indistinct, {110} indistinct |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 2.5-3 |
| Luster | Vitreous to pearly |
| Diaphaneity | Transparent |
| Specific gravity | 2.23 |
| Optical properties | Biaxial (+), Colorless (transmitted light) |
| Solubility | Insoluble in water, soluble in acids |
| References | [1][2][3][4] |
Whewellite, or at least crystalline calcium oxalate, does also arise from biological sources. Small crystals or flakes of it are sometimes found on the surfaces of some cacti, and kidney stones frequently have the same composition.
Whewellite was named after William Whewell (1794–1866), an English polymath, naturalist and scientist, professor of moral philosophy at Cambridge and inventor of the system of crystallographic indexing.
Heat decomposition
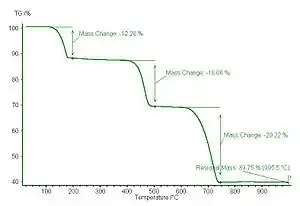
Whewellite is used as a thermogravimetric analysis standard due to its well-known decomposition temperatures and products.
References
- Whewellite: mindat.org
- Webmineral.com
- Handbook of Mineralogy
- Tazzoli, V.; Domeneghetti, M.C. (1980). "The crystal structures of whewellite and weddellite: re-examination and comparison". American Mineralogist. 65: 327–334. Retrieved 31 December 2020.
| Wikimedia Commons has media related to Whewellite. |
Bibliography
- Palache, P.; Berman H.; Frondel, C. (1960). "Dana's System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, Etc. (Seventh Edition)" John Wiley and Sons, Inc., New York, pp. 1099-1101.