Zincke reaction
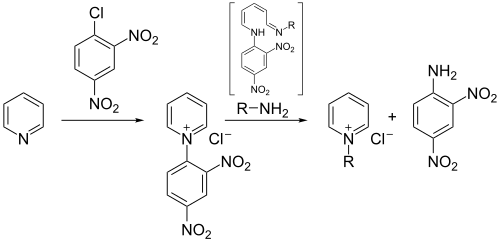
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke.[1][2][3]
| Zincke reaction | |
|---|---|
| Named after | Theodor Zincke |
| Reaction type | Coupling reaction |
The Zincke reaction should not be confused with the Zincke-Suhl reaction or the Zincke nitration. Furthermore, the Zincke reaction has nothing to do with the chemical element zinc.
Reaction mechanism
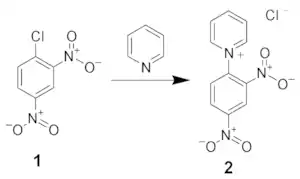
The first reaction is the formation of the N-2,4-dinitrophenyl-pyridinium salt (2). This salt is typically isolated and purified by recrystallization.
Upon heating a primary amine with the N-2,4-dinitrophenyl-pyridinium salt (2), the addition of the amine leads to the opening of the pyridinium ring. A second addition of amine leads to the displacement of 2,4-dinitroaniline (5) and formation of the König salt [4] (6a and 6b). The trans-cis-trans isomer of the König salt (6a) can react by either sigmatropic rearrangement or nucleophilic addition of a zwitterionic intermediate to give cyclized intermediate (7).[5] This has been suggested to be the rate-determining step.[6][7] After proton transfer and amine elimination, the desired pyridinium ion (9) is formed.
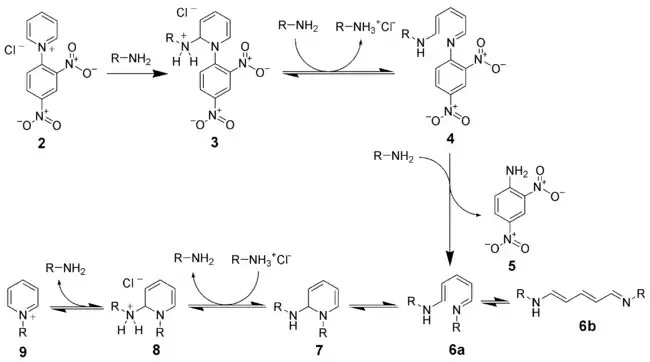
This mechanism can be referred to as an instance of the ANRORC mechanism: nucleophilic addition (AN), ring opening and ring closing.
Applications
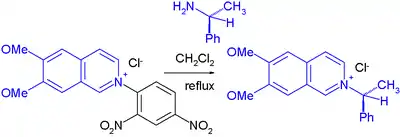
In one solid-phase synthesis application, the amine is covalently attached to Wang resin.[8]
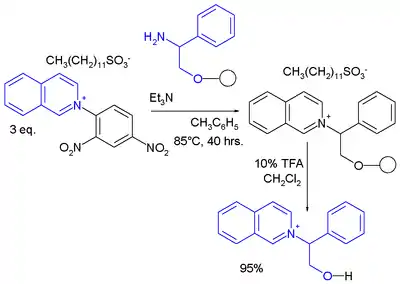
Another example is the synthesis of a chiral isoquinolinium salt.[9]
Zincke aldehydes
With secondary amines and not primary amines the Zincke reaction takes on a different shape forming so-called Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde:[10]
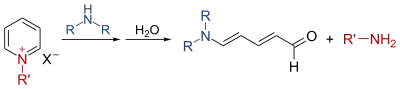
This variation has been applied in the synthesis of novel indoles:[11]

with cyanogen bromide mediated pyridine activation.
2007 rediscovery
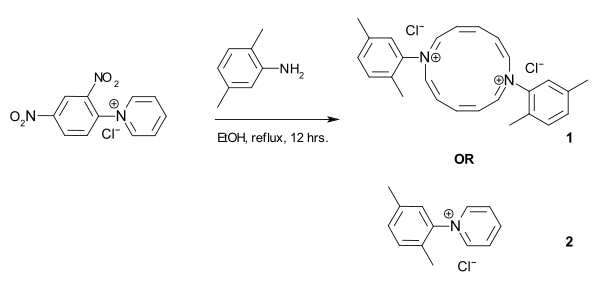
In 2006 and again in 2007 the Zincke reaction was rediscovered by a research group from Japan [12] and a group from the USA.[13] Both groups claimed the synthesis of a 12 membered diazaannulene (structure 1) from an N-aryl pyridinium chloride and an amine, an aniline in the case of the Japanese group (depicted below) and an aliphatic amine (anticipating surfactant properties) in the case of the American group.
In a letter to Angewandte Chemie, the German chemist Manfred Christl [14] pointed out not only that the alleged new chemistry was in fact 100-year-old Zincke chemistry but also that the proposed structure for the reaction product was not the 12 membered ring but the 6 membered pyridinium salt (structure 2). Initially both groups conceded that they had ignored existing literature on Zincke but held on to the annulene structure based on their electrospray ionization (ESI) results which according to them clearly showed dimer. In his letter Christl remarked that in ESI measurements association of molecules is a common phenomenon. In addition, he noted similarities in melting point and NMR spectroscopy.
As of December 2007 the Japanese group retracted its paper in Organic Letters due to uncertainties regarding what products are formed in the reaction described and the US group added a correction to theirs in the Angewandte Chemie stating they wish(ed) to alter the proposed structure of (the) annulene.[15] The issue did receive some media coverage:[16][17]
References
- Zincke, Th.; Heuser, G.; Moller, W. (1904). "Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte". Justus Liebigs Annalen der Chemie. 333 (2–3): 296–345. doi:10.1002/jlac.19043330212.CS1 maint: multiple names: authors list (link)
- Zincke, Th.; Heuser, G.; Moller, W. (1904). "Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte". Justus Liebigs Annalen der Chemie. 330 (2): 361–374. doi:10.1002/jlac.19043300217.CS1 maint: multiple names: authors list (link)
- Zincke, T. H.; Weisspfenning, G. (1913). "Über Dinitrophenylisochinoliniumchlorid und dessen Umwandlungsprodukte". Justus Liebigs Annalen der Chemie. 396 (1): 103–131. doi:10.1002/jlac.19133960107.
- König, W. (1904). "Über eine neue, vom Pyridin derivierende Klasse von Farbstoffen". Journal für Praktische Chemie. 69 (1): 105–137. doi:10.1002/prac.19040690107.
- Kunugi, S.; Okubo, T.; Ise, N. (1976). "A study on the mechanism of the reaction of N-(2,4-dinitrophenyl)-3-carbamoylpyridinium chloride with amines and amino acids with reference to effect of polyelectrolyte addition". Journal of the American Chemical Society. 98 (1): 2282–2287. doi:10.1021/ja00424a047. PMID 1254864.
- Marvell, E. N.; Caple, G.; Shahidi, I. J. Am. Chem. Soc. 1970, 92, 5641-5645. (doi:10.1021/ja00722a016)
- Marvell, E. N.; Shahidi, I. J. Am. Chem. Soc. 1970, 92, 5646-5649. (doi:10.1021/ja00722a017)
- "The Solid-Phase Zincke Reaction: Preparation of -Hydroxy Pyridinium Salts in the Search for CFTR Activation" Eda, M.; Kurth, M. J.; Nantz, M. H. J. Org. Chem. 2000, 65(17), 5131 - 5135. (doi:10.1021/jo0001636)
- New Chiral Isoquinolinium Salt Derivatives from Chiral Primary Amines via Zincke Reaction Denis Barbier, Christian Marazano, Bhupesh C. Das, and Pierre Potier J. Org. Chem.; 1996; 61(26) pp 9596 - 9598; (Note) doi:10.1021/jo961539b
- T. Zincke, W. Wurker, Justus Liebigs Ann. Chem. Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte, 1904, 338, 107 – 141; doi:10.1002/jlac.19043380107
- Synthesis of Nitrogen Heterocycles by the Ring Opening of Pyridinium Salts Aaron M. Kearney, Christopher D. Vanderwal Angew. Chem. Int. Ed. 2006, 45, 7803 –7806 doi:10.1002/anie.200602996
- One-Pot Synthesis of N-Substituted Diaza[12]annulenes Yamaguchi, I.; Gobara, Y.; Sato, M. Org. Lett.; (Letter); 2006; 8(19); 4279-4281. doi:10.1021/ol061585q
- [12]Annulene Gemini Surfactants: Structure and Self-Assembly Lei Shi, Dan Lundberg, Djamaladdin G. Musaev, Fredric M. Menger Angewandte Chemie International Edition Volume 46, 2007 Issue 31 , Pages 5889 - 5891 doi:10.1002/anie.200702140
- (in German) 1,7-Diaza[12]annulene Derivatives? 100-Year-Old Pyridinium Salts! (p 9152-9153)Manfred Christl Published Online: Nov 28 2007 5:47AM doi:10.1002/anie.200704704
- Corrigendum [12]Annulene Gemini Surfactants: Structure and Self-Assembly Lei Shi, Dan Lundberg, Djamaladdin G. Musaev, Fredric M. Menger Angewandte Chemie International Edition Volume 46, 2007, Issue 48, Pages 9152 - 9153 doi:10.1002/anie.200790248
- (in German) Ahnungslose Chemiker entdecken Verbindung zum zweiten Mal. Jens Lubbadeh Der Spiegel 6 December 2007 http://www.spiegel.de/wissenschaft/natur/0,1518,521646,00.html
- Sanderson, Katharine (4 December 2007). "Where have I seen that before? 103-year-old chemical reaction pops up again". Nature. doi:10.1038/news.2007.341.