(Diacetoxyiodo)benzene
(Diacetoxyiodo)benzene, also known as phenyliodine(III) diacetate (PIDA) is a hypervalent iodine chemical with the formula C
6H
5I(OCOCH
3)
2. It is used as an oxidizing agent in organic chemistry.
benzene.svg.png.webp) | |
| Names | |
|---|---|
| IUPAC name
[acetyloxy(phenyl)-λ3-iodanyl] acetate | |
| Other names
Bis(acetoxy)(phenyl)iodane Bis(acetato-O)phenyliodine Bis(acetoxy)iodobenzene (BAIB) (Diacetoxyiodo)benzene I,I-Diacetatoiodobenzene Iodobenzene diacetate Iodosobenzene I,I-diacetate Phenyliodine(III) diacetate (PIDA) Phenyliodo diacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.826 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H11IO4 | |
| Molar mass | 322.098 g·mol−1 |
| Appearance | white powder |
| Melting point | 163–165 °C (325–329 °F; 436–438 K) |
| reacts | |
| Solubility | soluble in acetic acid, acetonitrile, dichloromethane |
| Structure[1][2] | |
| orthorhombic | |
| Pnn2 | |
| T-shaped molecular geometry | |
| Related compounds | |
Related compounds |
(Bis(trifluoroacetoxy)iodo)benzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
This reagent was originally prepared by Conrad Willgerodt[3] by reacting iodobenzene with a mixture of acetic acid and peracetic acid:[4][5]
PIDA can also be prepared from iodosobenzene and glacial acetic acid:[5]
More recent preparations direct from iodine, acetic acid, and benzene have been reported, using either sodium perborate[6] or potassium peroxydisulfate[7] as the oxidizing agent:[8]
The PIDA molecule is termed hypervalent as its iodine atom (technically a hypervalent iodine) is in its +III oxidation state and has more than typical number of covalent bonds.[9] It adopts a T-shaped molecular geometry, with the phenyl group occupying one of the three equatorial positions of a trigonal bipyramid (lone pairs occupy the other two) and the axial positions occupied by oxygen atoms from the acetate groups. The "T" is distorted in that the phenyl-C to I to acetate-O bond angles are less than 90°.[1] A separate investigation of the crystal structure confirmed that it has orthorhombic crystals in space group Pnn2 and reported unit-cell dimensions in good agreement with the original paper.[1][2] The bond lengths around the iodine atom were 2.08 Å to the phenyl carbon atom and equal 2.156 Å bonds to the acetate oxygen atoms. This second crystal structure determination explained the distortion in the geometry by noting the presence of two weaker intramolecular iodine–oxygen interactions, resulting in an "overall geometry of each iodine [that] can be described as a pentagonal-planar arrangement of three strong and two weak secondary bonds."[2]
Unconventional reactions
One use of PIDA is in the preparation of similar reagents by substitution of the acetate groups. For example, it can be used to prepare (bis(trifluoroacetoxy)iodo)benzene (phenyliodine(III) bis(trifluoroacetate), PIFA) by heating in trifluoroacetic acid:[10][8]
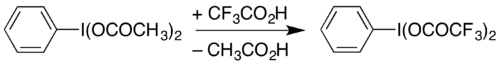
PIFA can be used to carry out the Hofmann rearrangement under mildly acidic conditions,[11] rather than the strongly basic conditions traditionally used.[12][13] The Hofmann decarbonylation of an N-protected asparagine has been demonstrated with PIDA, providing a route to β-amino-L-alanine derivatives.[14]
References
- Lee, Chow-Kong; Mak, Thomas C. W.; Li, Wai-Kee; Kirner, John F. (1977). "Iodobenzene diacetate". Acta Crystallogr. B. 33 (5): 1620–1622. doi:10.1107/S0567740877006694.
- Alcock, Nathaniel W.; Countryman, Rachel M.; Esperås, Steinar; Sawyer, Jeffery F. (1979). "Secondary bonding. Part 5. The crystal and molecular structures of phenyliodine(III) diacetate and bis(dichloroacetate)". J. Chem. Soc., Dalton Trans. 1979 (5): 854–860. doi:10.1039/DT9790000854.
- Willgerodt, C. (1892). "Zur Kenntniss aromatischer Jodidchloride, des Jodoso- und Jodobenzols". Chem. Ber. (in German). 25 (2): 3494–3502. doi:10.1002/cber.189202502221.
- Sharefkin, J. G.; Saltzman, H. (1963). "Iodosobenzene Diacetate". Organic Syntheses. 43: 62. doi:10.15227/orgsyn.043.0062.; Collective Volume, 5, p. 660
- Moriarty, Robert M.; Chany, Calvin J.; Kosmeder, Jerome W.; Du Bois, Justin (2001). "(Diacetoxyiodo)benzene". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289x.rd005m.pub2. ISBN 9780470842898.
- Hossain, Md. Delwar; Kitamura, Tsugio (2005). "Unexpected, Drastic Effect of Triflic Acid on Oxidative Diacetoxylation of Iodoarenes by Sodium Perborate. A Facile and Efficient One-Pot Synthesis of (Diacetoxyiodo)arenes". J. Org. Chem. 70 (17): 6984–6986. doi:10.1021/jo050927n. PMID 16095332.
- Hossain, Md. Delwar; Kitamura, Tsugio (2007). "New and Direct Approach to Hypervalent Iodine Compounds from Arenes and Iodine. Straightforward Synthesis of (Diacetoxyiodo)arenes and Diaryliodonium Salts Using Potassium μ-Peroxo-hexaoxodisulfate". Bull. Chem. Soc. Jpn. 80 (11): 2213–2219. doi:10.1246/bcsj.80.2213.
- Dohi, Toshifumi; Kita, Yasuyuki (2015). "Oxidizing Agents". In Kaiho, Tatsuo (ed.). Iodine Chemistry and Applications. John Wiley & Sons. pp. 277–302. ISBN 9781118878651.
- Dohi, Toshifumi; Kita, Yasuyuki (2015). "Hypervalent Iodine". In Kaiho, Tatsuo (ed.). Iodine Chemistry and Applications. John Wiley & Sons. pp. 103–158. ISBN 9781118878651.
- Almond, M. R.; Stimmel, J. B.; Thompson, E. A.; Loudon, G. M. (1988). "Hofmann Rearrangement Under Mildly Acidic Conditions Using [I,I-Bis(Trifluoroacetoxy)]Iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide". Organic Syntheses. 66: 132. doi:10.15227/orgsyn.066.0132.; Collective Volume, 8, p. 132
- Aubé, Jeffrey; Fehl, Charlie; Liu, Ruzhang; McLeod, Michael C.; Motiwala, Hashim F. (2014). "6.15 Hofmann, Curtius, Schmidt, Lossen, and Related Reactions". Heteroatom Manipulations. Comprehensive Organic Synthesis II. 6. pp. 598–635. doi:10.1016/B978-0-08-097742-3.00623-6. ISBN 9780080977430.
- Wallis, Everett S.; Lane, John F. (1946). "The Hofmann Reaction". Org. React. 3 (7): 267–306. doi:10.1002/0471264180.or003.07.
- Surrey, Alexander R. (1961). "Hofmann Reaction". Name Reactions in Organic Chemistry (2nd ed.). Academic Press. pp. 134–136. ISBN 9781483258683.
- Zhang, Lin-hua; Kauffman, Goss S.; Pesti, Jaan A.; Yin, Jianguo (1997). "Rearrangement of Nα-Protected L-Asparagines with Iodosobenzene Diacetate. A Practical Route to β-Amino-L-alanine Derivatives". J. Org. Chem. 62 (20): 6918–6920. doi:10.1021/jo9702756.