Affimer
Affimer molecules[1] are small proteins that bind to target molecules with similar specificity and affinity to that of antibodies.[2][3] These engineered non-antibody binding proteins are designed to mimic the molecular recognition characteristics of monoclonal antibodies in different applications.[2][4] In addition, these affinity reagents have been optimized to increase their stability,[5] make them tolerant to a range of temperatures and pH,[6] reduce their size, and to increase their expression in E.coli and mammalian cells.[2]
Development
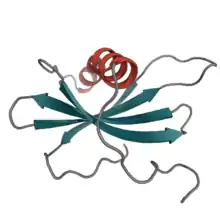
Affimer proteins were developed initially at the MRC Cancer Cell Unit in Cambridge then across two laboratories at the University of Leeds.[7][8][9][10] Derived from the cysteine protease inhibitor family of cystatins, which function in nature as cysteine protease inhibitors,[11][12] these 12–14 kDa proteins share the common tertiary structure of an alpha-helix lying on top of an anti-parallel beta-sheet.[13]
Affimer proteins display two peptide loops and an N-terminal sequence that can all be randomised to bind to desired target proteins with high affinity and specificity,[5] in a similar manner to monoclonal antibodies. Stabilisation of the two peptides by the protein scaffold constrains the possible conformations that the peptides can take, increasing the binding affinity and specificity compared to libraries of free peptides.
Production
Phage display libraries of 1010 randomised potential target interaction sequences[14] are used to screen for Affimer proteins that exhibit high-specificity binding to the target protein with binding affinities in the nM range.[5][15][16] The ability to direct in vitro screening techniques allows the identification of highly specific, high affinity Affimer binders, negating requirements for affinity maturation of these reagents. In vitro screening and development also mean that the target space for Affimer binders is not limited by an animal host's immune system. Due to Affimer proteins being generated using recombinant systems, their generation is significantly more rapid and reproducible compared to the production of traditional antibodies.[17]
Multimeric forms of Affimer proteins have been generated and shown to yield titrimetric volumes in the range of 200–400 mg/L under small scale culture using bacterial host systems. Multimeric forms of Affimer proteins with the same target specificity provided avidity effects in target binding, while fusion of different Affimer proteins with different target specificities would enable multi-specific affinity proteins.[18]
Many different tags and fusion proteins, such as fluorophores, single-stranded DNA, His, and c-Myc tags can be easily conjugated to Affimer proteins.[2][19][20][21][22][23] Specific cysteine residues can be introduced to the protein to allow thiol chemistry to uniformly orient Affimer proteins on a solid support for the purposes of increasing target capture in ligand binding assays and biosensors.[5][15][17][24][25] This flexible functionalisation of the Affimer molecule maximises the performance of Affimer reagents across multiple applications and assay formats.
Properties
Affimer binders are recombinant proteins. They display the robust characteristics of high thermostability, with melting temperatures over 80 °C,[10] resistance to extremes of pH (2–13.7),[10] freeze-thaw cycles and lyophilisation. The low molecular weight of Affimer binders means that problems of steric hindrance, that are typically observed with antibodies, may be avoided.[2] As they are manufactured using recombinant bacterial production processes, the batch-to-batch consistency is maintained for Affimer reagents, overcoming some of the issues of reproducibility and security of supply.[2][3][17][26][27]
These synthetic antibodies were engineered to be stable, non-toxic, biologically neutral and contain no post-translational modifications or disulfide bridges.[7][8][10] Two separate loop sequences, incorporating a total of 12 to 36 amino acids, form the target interaction surface so interaction surfaces can range form 650–1000 Å. The large interaction surface is purported to result in highly-specific, high affinity binding to target proteins.[5][7][10][15][17][19][28][29][30][31] As a result, Affimer molecules can distinguish between proteins that differ by only a single amino acid, can detect subtle changes in protein expression levels even in a multiplexed format and can distinguish between multiple closely related protein domains.[2][24][31][32][33]
Applications
Affimer technology has been commercialised and developed by Avacta, who are developing these affinity reagents as tools for research and diagnostics and as biotherapeutics.
Reagents and Diagnostics
Affimer binders have been used across a number of platforms, including ELISA,[2][17][34] surface plasmon resonance,[32][34][35][36] affinity purification,[2][34][37] immunohistochemistry[2] and immunocytochemistry, including super resolution imaging.[2][19][20][21] Affimer reagents that inhibit protein-protein interactions can also be produced with the potential to express these inhibitors in mammalian cells to investigate and modify signalling pathways.[2][32][35][38][39] They have also been co-crystallised in complex with target proteins,[32][35][40] enabling drug discovery through in silico screening and displacement assays.[14]
Affimer reagents are suitable for use in biosensors,[5][15][24][25] point-of-care diagnostics and as anti-idiotypic reagents in pharmacokinetic and therapeutic drug monitoring assays.[26][27][31][41][42]
Therapeutics
The Affimer protein scaffold has been developed as a biotherapeutic. The small size and stability profile of Affimer proteins combined with their human origin confer drug-like properties to Affimer molecules. This may represent advantages over antibodies in terms of tissue penetration, for example in solid tumours or for non-invasive topical administration, such as inhaled delivery or dermal application.[18]
Affimer proteins can be easily conjugated to form multimers and be easily functionalised for the design of therapeutics with specific desired characteristics. Examples include the production of multi-specific Affimer molecules to target and recruit specific cells, fusion to Fc fragments or albumin binders to tune their half-life in vivo and for use as the targeting moiety in chimeric receptors or modified to carry a toxin in Affimer-drug conjugates.[16][18][43]
Affimer therapeutics are in discovery and preclinical development to tackle blood clotting disorders, antibiotic resistance, phenotypic drug discovery models and cancer, both via CAR-T cell therapy and as immune checkpoint inhibitors.[18][44][45][46] Early studies using ex vivo human samples showed low immunogenicity associated with the Affimer scaffold, at levels comparable to a marketed antibody therapeutic.[47] Furthermore, initial preclinical studies showed good efficacy and tolerability of the anti-PDL1 immuno-oncology Affimer therapeutic in mice. It is anticipated that IND filing for the first Affimer therapeutic will occur in 2019/2020.[16]
References
- Proprietary name, owned by Avacta
- Tiede C, Bedford R, Heseltine SJ, Smith G, Wijetunga I, Ross R, et al. (June 2017). "Affimer proteins are versatile and renewable affinity reagents". eLife. 6. doi:10.7554/elife.24903. PMC 5487212. PMID 28654419.
- Klont F, Hadderingh M, Horvatovich P, Ten Hacken NH, Bischoff R (August 2018). "Affimers as an Alternative to Antibodies in an Affinity LC-MS Assay for Quantification of the Soluble Receptor of Advanced Glycation End-Products (sRAGE) in Human Serum". Journal of Proteome Research. 17 (8): 2892–2899. doi:10.1021/acs.jproteome.8b00414. PMC 6079930. PMID 30005571.
- "Antibody Alternatives". The Scientist Magazine®. Retrieved 2018-10-18.
- Sharma R, Deacon SE, Nowak D, George SE, Szymonik MP, Tang AA, Tomlinson DC, Davies AG, McPherson MJ, Wälti C (June 2016). "Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/ml sensitivity". Biosensors & Bioelectronics. 80: 607–613. doi:10.1016/j.bios.2016.02.028. PMC 4785862. PMID 26897263.
- Rawlings AE, Bramble JP, Tang AA, Somner LA, Monnington AE, Cooke DJ, McPherson MJ, Tomlinson DC, Staniland SS (October 2015). "Phage display selected magnetite interacting Adhirons for shape controlled nanoparticle synthesis". Chemical Science. 6 (10): 5586–5594. doi:10.1039/C5SC01472G. PMC 5949846. PMID 29861896.
- Woodman R, Yeh JT, Laurenson S, Ko Ferrigno P (October 2005). "Design and validation of a neutral protein scaffold for the presentation of peptide aptamers". Journal of Molecular Biology. 352 (5): 1118–33. doi:10.1016/j.jmb.2005.08.001. PMID 16139842.
- Hoffmann T, Stadler LK, Busby M, Song Q, Buxton AT, Wagner SD, Davis JJ, Ko Ferrigno P (May 2010). "Structure-function studies of an engineered scaffold protein derived from stefin A. I: Development of the SQM variant". Protein Engineering, Design & Selection. 23 (5): 403–13. doi:10.1093/protein/gzq012. PMC 2851446. PMID 20179045.
- Stadler LK, Hoffmann T, Tomlinson DC, Song Q, Lee T, Busby M, Nyathi Y, Gendra E, Tiede C, Flanagan K, Cockell SJ, Wipat A, Harwood C, Wagner SD, Knowles MA, Davis JJ, Keegan N, Ferrigno PK (September 2011). "Structure-function studies of an engineered scaffold protein derived from Stefin A. II: Development and applications of the SQT variant". Protein Engineering, Design & Selection. 24 (9): 751–63. doi:10.1093/protein/gzr019. PMID 21616931.
- Tiede C, Tang AA, Deacon SE, Mandal U, Nettleship JE, Owen RL, George SE, Harrison DJ, Owens RJ, Tomlinson DC, McPherson MJ (May 2014). "Adhiron: a stable and versatile peptide display scaffold for molecular recognition applications". Protein Engineering, Design & Selection. 27 (5): 145–55. doi:10.1093/protein/gzu007. PMC 4000234. PMID 24668773.
- Turk V, Stoka V, Turk D (May 2008). "Cystatins: biochemical and structural properties, and medical relevance". Frontiers in Bioscience. 13 (13): 5406–20. doi:10.2741/3089. PMID 18508595.
- Kondo H, Abe K, Emori Y, Arai S (January 1991). "Gene organization of oryzacystatin-II, a new cystatin superfamily member of plant origin, is closely related to that of oryzacystatin-I but different from those of animal cystatins". FEBS Letters. 278 (1): 87–90. doi:10.1016/0014-5793(91)80090-p. PMID 1993479.
- Turk V, Bode W (July 1991). "The cystatins: protein inhibitors of cysteine proteinases". FEBS Letters. 285 (2): 213–9. doi:10.1016/0014-5793(91)80804-C. PMID 1855589.
- Arrata I, Barnard A, Tomlinson DC, Wilson AJ (March 2017). "Interfacing native and non-native peptides: using Affimers to recognise α-helix mimicking foldamers" (PDF). Chemical Communications. 53 (19): 2834–2837. doi:10.1039/c6cc09395g. PMID 28217789.
- Zhurauski P, Arya SK, Jolly P, Tiede C, Tomlinson DC, Ko Ferrigno P, Estrela P (June 2018). "Sensitive and selective Affimer-functionalised interdigitated electrode-based capacitive biosensor for Her4 protein tumour biomarker detection" (PDF). Biosensors & Bioelectronics. 108: 1–8. doi:10.1016/j.bios.2018.02.041. PMID 29482002.
- Basran A, Stanley E (2018-07-01). "Abstract 3776: Generation and formatting of an Affimer® biotherapeutic for the inhibition of the PD-L1/PD-1 pathway: Proof of concept in mouse". Cancer Research. 78 (13 Supplement): 3776. doi:10.1158/1538-7445.AM2018-3776. ISSN 0008-5472.
- Xie C, Tiede C, Zhang X, Wang C, Li Z, Xu X, McPherson MJ, Tomlinson DC, Xu W (August 2017). "Development of an Affimer-antibody combined immunological diagnosis kit for glypican-3". Scientific Reports. 7 (1): 9608. doi:10.1038/s41598-017-10083-w. PMC 5575301. PMID 28852111.
- "Interview with Amrik Basran at Avacta Life Sciences". Drug Target Review.
- Schlichthaerle T, Eklund AS, Schueder F, Strauss MT, Tiede C, Curd A, Ries J, Peckham M, Tomlinson DC, Jungmann R (August 2018). "Site-Specific Labeling of Affimers for DNA-PAINT Microscopy" (PDF). Angewandte Chemie. 57 (34): 11060–11063. doi:10.1002/anie.201804020. PMID 29873161.
- Bedford R, Tiede C, Hughes R, Curd A, McPherson MJ, Peckham M, Tomlinson DC (August 2017). "Alternative reagents to antibodies in imaging applications". Biophysical Reviews. 9 (4): 299–308. doi:10.1007/s12551-017-0278-2. PMC 5578921. PMID 28752365.
- Lopata A, Hughes R, Tiede C, Heissler SM, Sellers JR, Knight PJ, Tomlinson D, Peckham M (April 2018). "Affimer proteins for F-actin: novel affinity reagents that label F-actin in live and fixed cells". Scientific Reports. 8 (1): 6572. doi:10.1038/s41598-018-24953-4. PMC 5920084. PMID 29700342.
- Wang W, Guo Y, Tiede C, Chen S, Kopytynski M, Kong Y, Kulak A, Tomlinson D, Chen R, McPherson M, Zhou D (May 2017). "Ultraefficient Cap-Exchange Protocol To Compact Biofunctional Quantum Dots for Sensitive Ratiometric Biosensing and Cell Imaging". ACS Applied Materials & Interfaces. 9 (18): 15232–15244. doi:10.1021/acsami.6b13807. PMC 5432960. PMID 28421739.
- Fisher MJ, Williamson DJ, Burslem GM, Plante JP, Manfield IW, Tiede C, Ault JR, Stockley PG, Plein S, Maqbool A, Tomlinson DC, Foster R, Warriner SL, Bon RS (December 2015). "Trivalent Gd-DOTA reagents for modification of proteins". RSC Advances. 5 (116): 96194–96200. doi:10.1039/c5ra20359g. PMC 4786947. PMID 27019702.
- Weckman NE, McRae C, Ko Ferrigno P, Seshia AA (October 2016). "Comparison of the specificity and affinity of surface immobilised Affimer binders using the quartz crystal microbalance". The Analyst. 141 (22): 6278–6286. doi:10.1039/c6an01602b. PMID 27704086.
- Koutsoumpeli E, Tiede C, Murray J, Tang A, Bon RS, Tomlinson DC, Johnson S (March 2017). "Antibody Mimetics for the Detection of Small Organic Compounds Using a Quartz Crystal Microbalance". Analytical Chemistry. 89 (5): 3051–3058. doi:10.1021/acs.analchem.6b04790. PMID 28192970.
- "Anti-idiotypic binders for Trastuzumab validated in regulatory bioanalysis assay in collaboration with Covance". Avacta. Retrieved 2018-10-19.
- "Development and validation of anti-idiotypic Affmers to trastuzumab in a pharmacokinetic assay | Avacta". www.avacta.com. Retrieved 2018-10-19.
- Davis JJ, Tkac J, Laurenson S, Ko Ferrigno P (February 2007). "Peptide aptamers in label-free protein detection: 1. Characterization of the immobilized scaffold". Analytical Chemistry. 79 (3): 1089–96. doi:10.1021/ac061863z. PMID 17263340.
- Davis JJ, Tkac J, Humphreys R, Buxton AT, Lee TA, Ko Ferrigno P (May 2009). "Peptide aptamers in label-free protein detection: 2. Chemical optimization and detection of distinct protein isoforms". Analytical Chemistry. 81 (9): 3314–20. doi:10.1021/ac802513n. PMID 19320493.
- "Affimer binders improve diagnostic assay performance for C.difficile detection". Avacta. Retrieved 2018-10-19.
- "Development and characterisation of Affimer binders to 4 leading therapeutic antibodies | Avacta". www.avacta.com. Retrieved 2018-10-19.
- Robinson JI, Baxter EW, Owen RL, Thomsen M, Tomlinson DC, Waterhouse MP, Win SJ, Nettleship JE, Tiede C, Foster RJ, Owens RJ, Fishwick CW, Harris SA, Goldman A, McPherson MJ, Morgan AW (January 2018). "Affimer proteins inhibit immune complex binding to FcγRIIIa with high specificity through competitive and allosteric modes of action". Proceedings of the National Academy of Sciences of the United States of America. 115 (1): E72–E81. doi:10.1073/pnas.1707856115. PMC 5776790. PMID 29247053.
- Evans D, Johnson S, Laurenson S, Davies AG, Ko Ferrigno P, Wälti C (2008). "Electrical protein detection in cell lysates using high-density peptide-aptamer microarrays". Journal of Biology. 7 (1): 3. doi:10.1186/jbiol62. PMC 2246035. PMID 18237447.
- "Avacta Scientist Dr Geoff Platt presents Affimer data in webinar | Avacta". www.avacta.com. Retrieved 2018-10-22.
- Michel MA, Swatek KN, Hospenthal MK, Komander D (October 2017). "Ubiquitin Linkage-Specific Affimers Reveal Insights into K6-Linked Ubiquitin Signaling". Molecular Cell. 68 (1): 233–246.e5. doi:10.1016/j.molcel.2017.08.020. PMC 5640506. PMID 28943312.
- Johnson A, Song Q, Ko Ferrigno P, Bueno PR, Davis JJ (August 2012). "Sensitive affimer and antibody based impedimetric label-free assays for C-reactive protein". Analytical Chemistry. 84 (15): 6553–60. doi:10.1021/ac300835b. PMID 22789061.
- "Affimer® reagents facilitate affinity chromatography purification". www.drugtargetreview.com. Retrieved 2018-10-22.
- Kyle HF, Wickson KF, Stott J, Burslem GM, Breeze AL, Tiede C, Tomlinson DC, Warriner SL, Nelson A, Wilson AJ, Edwards TA (October 2015). "Exploration of the HIF-1α/p300 interface using peptide and Adhiron phage display technologies". Molecular BioSystems. 11 (10): 2738–49. doi:10.1039/c5mb00284b. PMID 26135796.
- Heidelberger JB, Voigt A, Borisova ME, Petrosino G, Ruf S, Wagner SA, Beli P (April 2018). "Proteomic profiling of VCP substrates links VCP to K6-linked ubiquitylation and c-Myc function". EMBO Reports. 19 (4): e44754. doi:10.15252/embr.201744754. PMC 5891417. PMID 29467282.
- Gersch M, Gladkova C, Schubert AF, Michel MA, Maslen S, Komander D (November 2017). "Mechanism and regulation of the Lys6-selective deubiquitinase USP30". Nature Structural & Molecular Biology. 24 (11): 920–930. doi:10.1038/nsmb.3475. PMC 5757785. PMID 28945249.
- "Covance validate Affimers as critical PK assay reagents". Avacta. Retrieved 2018-10-22.
- "Webinar: Multiplexing Specificity and Species Cross Reactivity in Biologics Discovery (On-Demand) - Intellicyt". Intellicyt. Retrieved 2018-10-22.
- "TMAC Affimer Drug Conjugates". Avacta. Retrieved 2018-10-22.
- Mulligan, Tom. "Avacta and Memorial Sloan Kettering Cancer Center in cell therapy research collaboration". Retrieved 2018-10-22.
- "Pipeline | Avacta". www.avacta.com. Retrieved 2018-10-22.
- King R, Tiede C, Simmons K, Fishwick C, Tomlinson D, Ajjan R (February 2015). "Inhibition of complement C3 and fibrinogen interaction: a potential novel therapeutic target to reduce cardiovascular disease in diabetes". Lancet. 385 Suppl 1: S57. doi:10.1016/s0140-6736(15)60372-5. PMID 26312879.
- "Affimer Technology: Results of PBMC Immunogenicity Testing". Avacta. Retrieved 2018-10-22.