Bispecific monoclonal antibody
A bispecific monoclonal antibody (BsMAb, BsAb) is an artificial protein that can simultaneously bind to two different types of antigen. BsMabs can be manufactured in several structural formats, and current applications have been explored for cancer immunotherapy and drug delivery.[1]
Structural types and manufacturing methods
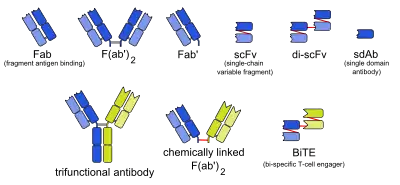
There are many formats of bsMab, but the two main categories are IgG-like and non-IgG-like.[1] The main types of manufacturing methods are quadromas, chemical conjugation, and genetic recombination, and each method results in a unique format.[1][2]
IgG-like
This format retains the traditional monoclonal antibody (mAb) structure of two Fab arms and one Fc region, except the two Fab sites bind different antigens. The most common types are called trifunctional antibodies, as they have three unique binding sites on the antibody: the two Fab regions, and the Fc region. Each heavy and light chain pair is from a unique mAb. The Fc region made from the two heavy chains forms the third binding site. These bsMabs are often manufactured with the quadroma, or the hybrid hybridoma, method.[3][4][5]
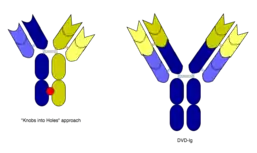
However, the quadroma method relies on random chance to form usable bsMabs, and can be inefficient. Another method for manufacturing IgG-like bsMabs is called "knobs into holes," and relies on introducing a mutation for a large amino acid in the heavy chain from one mAb, and a mutation for a small amino acid in the other mAb's heavy chain. This allows the target heavy chains (and their corresponding light chains) to fit together better, and makes bsMab production more reliable.[1][2]
Non-IgG-like
There are other bsMabs that lack an Fc region entirely. These include chemically linked Fabs, consisting of only the Fab regions, and various types of bivalent and trivalent single-chain variable fragments (scFvs). There are also fusion proteins mimicking the variable domains of two antibodies. The furthest developed of these newer formats are the bi-specific T-cell engagers (BiTEs).[6][7][8]
Mechanism of action
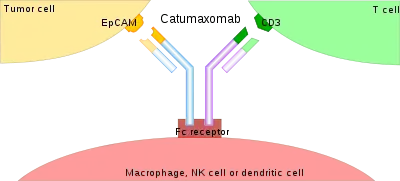
The binding of a bsMab to its target antigens can lead to a variety of effects. The most widely used application of this approach is in cancer immunotherapy, where bsMabs are engineered to simultaneously bind a cytotoxic cell and a target like a tumour cell to be destroyed. It is possible to observe the bridging effect that bsAbs have on T-cell/cancer cell interactions using label-free live cell imaging. Catumaxomab, one of the first trifunctional antibodies approved for therapeutic use, binds both CD3 on cytotoxic T cells and EpCAM on human adenocarcinomas.[3][4] The Fc region additionally binds to a cell that expresses Fc receptors, like a macrophage, natural killer cell or dendritic cell. Since the Fc region is still intact, this allows for the bsMab to trigger common immune responses when recognized by an Fc receptor, such as antibody-dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity.[5][7]
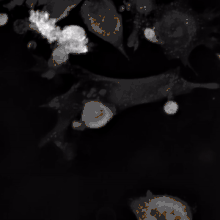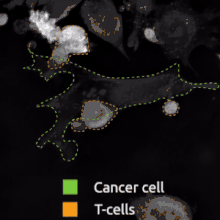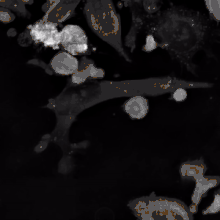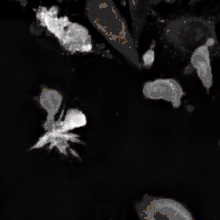
In work with Ebolavirus vaccines, a study has shown that a DVD-Ig antibody can be used to prevent viral escape from the endosome. Ebolaviruses infect cells by receptor-mediated endocytosis. Researchers developed DVD-Igs where the outer variable regions bind to the surface glycoproteins of the viral coat and enter the cell with the virus. These outer regions are cleaved in the viral endosome, revealing the inner variable regions that then bind to both the virus and internal receptors in the endosome. Blocking the interaction between the virus and endosomal proteins prevents viral escape from the endosome and further infection.[9]
Advantages over ordinary monoclonal antibodies
Cancer immunotherapy with ordinary monoclonal antibodies does not activate T-lymphocytes because the Fab regions are already used for binding the tumour cells, and this type of cell does not have Fc receptors.[10] Bispecific antibodies also have a higher cytotoxic potential, and bind to antigens that are expressed relatively weakly.[11] The effective dose is around 0.01 mg·m−2·d−1 (milligrams per square metre body surface area per day), which is several orders of magnitude lower than with ordinary antibodies.[10] For non-IgG-like bsMabs, their smaller size allows them to reach antigens usually unavailable to conventional antibodies.[1] In the case of Ebola vaccines, this method allows the antibody to target intracellular targets not usually accessible by traditional monoclonal antibody treatments.[9]
Additionally, targeting more than one molecule can be useful to circumvent the regulation of parallel pathways and avoid resistance to the treatment. Binding or blocking multiple targets in a pathway can be beneficial to stopping disease, as most conditions have complicated multifaceted effects throughout the body.[12] Together with combination therapies, bsMab are being used more and more to treat certain types of cancers, as, over time, some tumors develop resistances to checkpoint inhibitors and/or co-stimulatory molecules.[13]
Problems and current disadvantages
IgG-like antibodies can be immunogenic, which means the Fc region could cause detrimental downstream immune responses caused by cells that are activated by Fc receptors.[1] The therapeutic use of bsMabs as a whole is still largely in development, with many clinical trials currently ongoing that are determining the efficacy and safety of bsMabs for treatment.[6]
Clinical use
Two bispecific antibodies are presently in clinical use. Blinatumomab, which targets CD19 and CD3, is used in the treatment of Philadelphia chromosome negative B cell acute lymphoblastic leukemia (ALL). Emicizumab, which targets clotting factors IXa and X, is used in the treatment of hemophilia A.[14] Catumaxomab was withdrawn from the European market in 2017 for commercial reasons.[15]
References
![]() This article incorporates public domain material from the U.S. National Cancer Institute document: "Dictionary of Cancer Terms".
This article incorporates public domain material from the U.S. National Cancer Institute document: "Dictionary of Cancer Terms".
- Fan, Gaowei; Wang, Zujian; Hao, Mingju; Li, Jinming (2015-12-21). "Bispecific antibodies and their applications". Journal of Hematology & Oncology. 8: 130. doi:10.1186/s13045-015-0227-0. ISSN 1756-8722. PMC 4687327. PMID 26692321.
- Liu, Hongyan; Saxena, Abhishek; Sidhu, Sachdev S.; Wu, Donghui (2017-01-01). "Fc Engineering for Developing Therapeutic Bispecific Antibodies and Novel Scaffolds". Frontiers in Immunology. 8: 38. doi:10.3389/fimmu.2017.00038. PMC 5266686. PMID 28184223.
- Mueller, D; Kontermann, RE (2010). "Bispecific antibodies for cancer immunotherapy". BioDrugs. 24 (2): 89–98. doi:10.2165/11530960-000000000-00000. PMID 20199124.
- Chames, P; Baty, D (2009). "Bispecific antibodies for cancer therapy: The light at the end of the tunnel". mAbs. 1 (6): 539–547. doi:10.4161/mabs.1.6.10015. PMC 2791310. PMID 20073127.
- Lindhofer, H; Mocikat, R; Steipe, B; Thierfelder, S (1 July 1995). "Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies". Journal of Immunology. 155 (1): 219–25. PMID 7602098.
- Yang, Fa; Wen, Weihong; Qin, Weijun (2016-12-28). "Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies". International Journal of Molecular Sciences. 18 (1): 48. doi:10.3390/ijms18010048. PMC 5297683. PMID 28036020.
- Baeuerle, PA; Reinhardt, C (2009). "Bispecific T-cell engaging antibodies for cancer therapy". Cancer Res. 69 (12): 4941–4944. doi:10.1158/0008-5472.CAN-09-0547. PMID 19509221.
- Wozniak-Knopp, G.; Bartl, S.; Bauer, A.; Mostageer, M.; Woisetschlager, M.; Antes, B.; Ettl, K.; Kainer, M.; Weberhofer, G. (2010-04-01). "Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties". Protein Engineering Design and Selection. 23 (4): 289–297. doi:10.1093/protein/gzq005. ISSN 1741-0126. PMID 20150180.
- Wec, Anna Z.; Nyakatura, Elisabeth K.; Herbert, Andrew S.; Howell, Katie A.; Holtsberg, Frederick W.; Bakken, Russell R.; Mittler, Eva; Christin, John R.; Shulenin, Sergey (2016-10-21). "A "Trojan horse" bispecific-antibody strategy for broad protection against ebolaviruses". Science. 354 (6310): 350–354. Bibcode:2016Sci...354..350W. doi:10.1126/science.aag3267. ISSN 0036-8075. PMC 5647781. PMID 27608667.
- Bargou, R; Leo, E; Zugmaier, G; Klinger, M; Goebeler, M; Knop, S; Noppeney, R; Viardot, A; et al. (2008). "Tumor regression in cancer patients by very low doses of a T cell-engaging antibody". Science. 321 (5891): 974–977. Bibcode:2008Sci...321..974B. doi:10.1126/science.1158545. PMID 18703743.
- Weiner, LM; Holmes, M; Richeson, A; Godwin, A; Adams, GP; Hsieh-Ma, ST; Ring, DB; Alpaugh, RK (1993). "Binding and cytotoxicity characteristics of the bispecific murine monoclonal antibody 2B1". Journal of Immunology. 151 (5): 2877–86. PMID 8103070.
- Varela, MA (2015). "Identification of sequences common to more than one therapeutic target to treat complex diseases: simulating the high variance in sequence interactivity evolved to modulate robust phenotypes". BMC Genomics. 16: 530. doi:10.1186/s12864-015-1727-6. PMC 4506634. PMID 26187740.
- Koustas, E; Sarantis, P (2020). "The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors". Biomolecules. 10 (5): 66–82. doi:10.3390/biom10050666. PMID 32344837.
- Suurs, Frans V.; Lub-de Hooge, Marjolijn N.; de Vries, Elisabeth G. E.; de Groot, Derk Jan A. (2019-09-01). "A review of bispecific antibodies and antibody constructs in oncology and clinical challenges". Pharmacology & Therapeutics. 201: 103–119. doi:10.1016/j.pharmthera.2019.04.006. ISSN 0163-7258. PMID 31028837.
- "Removab: Withdrawal of the marketing authorisation in the European Union" (PDF). European Medicines Agency. 2017-07-10.
External links
- Bispecific monoclonal antibody entry in the public domain NCI Dictionary of Cancer Terms
- Bispecific+antibodies at the US National Library of Medicine Medical Subject Headings (MeSH)