Antimicrobial resistance
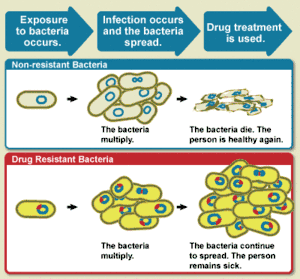
Antimicrobial resistance (AMR or AR) occurs when microbes evolve mechanisms that protect them from the effects of antimicrobials.[2] The term antibiotic resistance (AR or ABR) is a subset of AMR, as it applies to bacteria that become resistant to antibiotics.[2] Resistant microbes are more difficult to treat, requiring higher doses, or alternative medications which may prove more toxic. These approaches may also be more expensive. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR).
All classes of microbes can evolve resistance. Fungi evolve antifungal resistance. Viruses evolve antiviral resistance. Protozoa evolve antiprotozoal resistance, and bacteria evolve antibiotic resistance. Those bacteria that are considered extensively drug resistant (XDR) or totally drug-resistant (TDR) are sometimes called "superbugs".[3] Resistance in bacteria can arise naturally, by genetic mutation, or by one species acquiring resistance from another.[4] Resistance can appear spontaneously because of random mutations. However, extended use of antimicrobials appears to encourage selection for mutations which can render antimicrobials ineffective.
The prevention of antibiotic misuse which can lead to antibiotic resistance, includes prescribing or using antibiotics only when they are needed.[5][6] Narrow-spectrum antibiotics are preferred over broad-spectrum antibiotics when possible, as effectively and accurately targeting specific organisms is less likely to cause resistance, as well as side effects.[7][8] For people who take these medications at home, education about proper use is essential. Health care providers can minimize spread of resistant infections by use of proper sanitation and hygiene, including handwashing and disinfecting between patients, and should encourage the same of the patient, visitors, and family members.[9]
Rising drug resistance is caused mainly by use of antimicrobials in humans and other animals, and spread of resistant strains between the two.[5] Growing resistance has also been linked to dumping of inadequately treated effluents from the pharmaceutical industry, especially in countries where bulk drugs are manufactured.[10] Antibiotics increase selective pressure in bacterial populations, causing vulnerable bacteria to die; this increases the percentage of resistant bacteria which continue growing. Even at very low levels of antibiotic, resistant bacteria can have a growth advantage and grow faster than vulnerable bacteria.[11] With resistance to antibiotics becoming more common there is greater need for alternative treatments. Calls for new antibiotic therapies have been issued, but new drug development is becoming rarer.[12]
Antimicrobial resistance is increasing globally because of greater access to antibiotic drugs in developing countries.[13] Estimates are that 700,000 to several million deaths result per year and continues to pose a major public health threat worldwide.[14][15][16] Each year in the United States, at least 2.8 million people become infected with bacteria that are resistant to antibiotics and at least 35,000 people die as a result.[17] According to World Health Organization (WHO) estimates, three hundred and fifty million deaths could be caused by AMR by 2050.[18] By then, the yearly death toll will be ten million, according to a United Nations report.[19]
There are public calls for global collective action to address the threat that include proposals for international treaties on antimicrobial resistance.[20] Worldwide antibiotic resistance is not completely identified, but poorer countries with weaker healthcare systems are more affected.[6]
Definition
The WHO defines antimicrobial resistance as a microorganism's resistance to an antimicrobial drug that was once able to treat an infection by that microorganism.[2] A person cannot become resistant to antibiotics. Resistance is a property of the microbe, not a person or other organism infected by a microbe.[22]
Antibiotic resistance is a subset of antimicrobial resistance. This more specified resistance is linked to pathogenic bacteria and thus broken down into two further subsets, microbiological and clinical. Resistance linked microbiologically is the most common and occurs from genes, mutated or inherited, that allow the bacteria to resist the mechanism associated with certain antibiotics. Clinical resistance is shown through the failure of many therapeutic techniques where the bacteria that are normally susceptible to a treatment become resistant after surviving the outcome of the treatment. In both cases of acquired resistance, the bacteria can pass the genetic catalyst for resistance through conjugation, transduction, or transformation. This allows the resistance to spread across the same pathogen or even similar bacterial pathogens.[23]
Overview
WHO report released April 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance—when bacteria change so antibiotics no longer work in people who need them to treat infections—is now a major threat to public health."[24] In 2018, WHO considered antibiotic resistance to be one of the biggest threats to global health, food security and development.[25] The European Centre for Disease Prevention and Control calculated that in 2015 there were 671,689 infections in the EU and European Economic Area caused by antibiotic-resistant bacteria, resulting in 33,110 deaths. Most were acquired in healthcare settings.[26]
Causes
Antimicrobial resistance is mainly caused by the overuse of antimicrobials. This leads to microbes either evolving a defense against drugs used to treat them, or certain strains of microbes that have a natural resistance to antimicrobials becoming much more prevalent than the ones that are easily defeated with medication.[27] While antimicrobial resistance does occur naturally over time, the use of antimicrobial agents in a variety of settings both within the healthcare industry and outside of has led to antimicrobial resistance becoming increasingly more prevalent.[28]
Natural occurrence
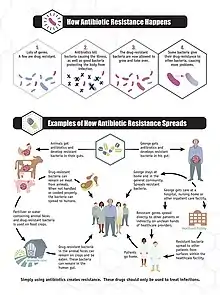
Antimicrobial resistance can evolve naturally due to continued exposure to antimicrobials. Natural selection means that organisms that are able to adapt to their environment survive and continue to produce offspring.[29] As a result, the types of microorganisms that are able to survive over time with continued attack by certain antimicrobial agents will naturally become more prevalent in the environment, and those without this resistance will become obsolete.[28] Over time most of the strains of bacteria and infections present will be the type resistant to the antimicrobial agent being used to treat them, making this agent now ineffective to defeat most microbes. With the increased use of antimicrobial agents, there is a speeding up of this natural process.[30]
Self medication
Self medication by consumers is defined as "the taking of medicines on one's own initiative or on another person's suggestion, who is not a certified medical professional", and it has been identified as one of the primary reasons for the evolution of antimicrobial resistance.[31] In an effort to manage their own illness, patients take the advice of false media sources, friends, and family causing them to take antimicrobials unnecessarily or in excess. Many people resort to this out of necessity, when they have a limited amount of money to see a doctor, or in many developing countries a poorly developed economy and lack of doctors are the cause of self-medication. In these developing countries, governments resort to allowing the sale of antimicrobials as over the counter medications so people could have access to them without having to find or pay to see a medical professional.[32] This increased access makes it extremely easy to obtain antimicrobials without the advice of a physician, and as a result many antimicrobials are taken incorrectly leading to resistant microbial strains. One major example of a place that faces these challenges is India, where in the state of Punjab 73% of the population resorted to treating their minor health issues and chronic illnesses through self-medication.[31]
The major issue with self-medication is the lack of knowledge of the public on the dangerous effects of antimicrobial resistance, and how they can contribute to it through mistreating or misdiagnosing themselves. In order to determine the public's knowledge and preconceived notions on antibiotic resistance, a major type of antimicrobial resistance, a screening of 3537 articles published in Europe, Asia, and North America was done. Of the 55,225 total people surveyed, 70% had heard of antibiotic resistance previously, but 88% of those people thought it referred to some type of physical change in the body.[31] With so many people around the world with the ability to self-medicate using antibiotics, and a vast majority unaware of what antimicrobial resistance is, it makes the increase of antimicrobial resistance much more likely.
Clinical misuse
Clinical misuse by healthcare professionals is another cause leading to increased antimicrobial resistance. Studies done by the CDC show that the indication for treatment of antibiotics, choice of the agent used, and the duration of therapy was incorrect in up to 50% of the cases studied. In another study done in an intensive care unit in a major hospital in France, it was shown that 30% to 60% of prescribed antibiotics were unnecessary.[33] These inappropriate uses of antimicrobial agents promote the evolution of antimicrobial resistance by supporting the bacteria in developing genetic alterations that lead to resistance.[34] In a study done by the American Journal of Infection Control aimed to evaluate physicians’ attitudes and knowledge on antimicrobial resistance in ambulatory settings, only 63% of those surveyed reported antibiotic resistance as a problem in their local practices, while 23% reported the aggressive prescription of antibiotics as necessary to avoid failing to provide adequate care.[35] This demonstrates how a majority of doctors underestimate the impact that their own prescribing habits have on antimicrobial resistance as a whole. It also confirms that some physicians may be overly cautious when it comes to prescribing antibiotics for both medical or legal reasons, even when indication for use for these medications is not always confirmed. This can lead to unnecessary antimicrobial use.
Environmental pollution
Untreated effluents from pharmaceutical manufacturing industries,[36] hospitals and clinics, and inappropriate disposal of unused or expired medication can expose microbes in the environment to antibiotics and trigger the evolution of resistance.
Livestock
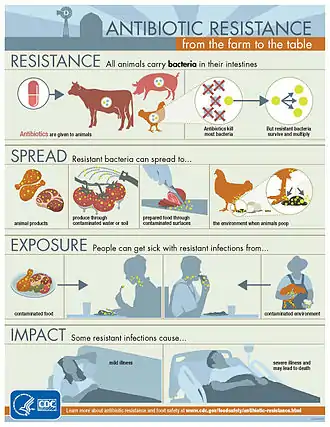
The antimicrobial resistance crisis also extends to the food industry, specifically with food producing animals. Antibiotics are fed to livestock to act as growth supplements, and a preventative measure to decrease the likelihood of infections. This results in the transfer of resistant bacterial strains into the food that humans eat, causing potentially fatal transfer of disease. While this practice does result in better yields and meat products, it is a major issue in terms of preventing antimicrobial resistance.[37] Though the evidence linking antimicrobial usage in livestock to antimicrobial resistance is limited, the World Health Organization Advisory Group on Integrated Surveillance of Antimicrobial Resistance strongly recommended the reduction of use of medically important antimicrobials in livestock. Additionally, the Advisory Group stated that such antimicrobials should be expressly prohibited for both growth promotion and disease prevention.[38]
In a study published by the National Academy of Sciences mapping antimicrobial consumption in livestock globally, it was predicted that in the 228 countries studied, there would be a total 67% increase in consumption of antibiotics by livestock by 2030. In some countries such as Brazil, Russia, India, China, and South Africa it is predicted that a 99% increase will occur.[30] Several countries have restricted the use of antibiotics in livestock, including Canada, China, Japan, and the US. These restrictions are sometimes associated with a reduction of the prevalence of antimicrobial resistance in humans.[38]
Pesticides
Most pesticides protect crops against insects and plants, but in some cases antimicrobial pesticides are used to protect against various microorganisms such as bacteria, viruses, fungi, algae, and protozoa. The overuse of many pesticides in an effort to have a higher yield of crops has resulted in many of these microbes evolving a tolerance against these antimicrobial agents. Currently there are over 4000 antimicrobial pesticides registered with the EPA and sold to market, showing the widespread use of these agents.[39] It is estimated that for every single meal a person consumes, 0.3 g of pesticides is used, as 90% of all pesticide use is used on agriculture. A majority of these products are used to help defend against the spread of infectious diseases, and hopefully protect public health. But out of the large amount of pesticides used, it is also estimated that less than 0.1% of those antimicrobial agents, actually reach their targets. That leaves over 99% of all pesticides used available to contaminate other resources.[40] In soil, air, and water these antimicrobial agents are able to spread, coming in contact with more microorganisms and leading to these microbes evolving mechanisms to tolerate and further resist pesticides.
Prevention
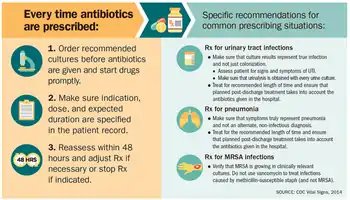
There have been increasing public calls for global collective action to address the threat, including a proposal for international treaty on antimicrobial resistance. Further detail and attention is still needed in order to recognize and measure trends in resistance on the international level; the idea of a global tracking system has been suggested but implementation has yet to occur. A system of this nature would provide insight to areas of high resistance as well as information necessary for evaluating programs and other changes made to fight or reverse antibiotic resistance.
Duration of antibiotics
Antibiotic treatment duration should be based on the infection and other health problems a person may have.[7] For many infections once a person has improved there is little evidence that stopping treatment causes more resistance.[7] Some therefore feel that stopping early may be reasonable in some cases.[7] Other infections, however, do require long courses regardless of whether a person feels better.[7]
Monitoring and mapping
There are multiple national and international monitoring programs for drug-resistant threats, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended spectrum beta-lactamase (ESBL), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Acinetobacter baumannii (MRAB).[41]
ResistanceOpen is an online global map of antimicrobial resistance developed by HealthMap which displays aggregated data on antimicrobial resistance from publicly available and user submitted data.[42][43] The website can display data for a 25-mile radius from a location. Users may submit data from antibiograms for individual hospitals or laboratories. European data is from the EARS-Net (European Antimicrobial Resistance Surveillance Network), part of the ECDC.
ResistanceMap is a website by the Center for Disease Dynamics, Economics & Policy and provides data on antimicrobial resistance on a global level.[44]
Limiting antibiotic use
Antibiotic stewardship programmes appear useful in reducing rates of antibiotic resistance.[45] The antibiotic stewardship program will also provide pharmacists with the knowledge to educate patients that antibiotics will not work for a virus.[46]
Excessive antibiotic use has become one of the top contributors to the evolution of antibiotic resistance. Since the beginning of the antibiotic era, antibiotics have been used to treat a wide range of disease.[47] Overuse of antibiotics has become the primary cause of rising levels of antibiotic resistance. The main problem is that doctors are willing to prescribe antibiotics to ill-informed individuals who believe that antibiotics can cure nearly all illnesses, including viral infections like the common cold. In an analysis of drug prescriptions, 36% of individuals with a cold or an upper respiratory infection (both viral in origin) were given prescriptions for antibiotics.[48] These prescriptions accomplished nothing other than increasing the risk of further evolution of antibiotic resistant bacteria.[49]
At the hospital level
Antimicrobial stewardship teams in hospitals are encouraging optimal use of antimicrobials.[50] The goals of antimicrobial stewardship are to help practitioners pick the right drug at the right dose and duration of therapy while preventing misuse and minimizing the development of resistance. Stewardship may reduce the length of stay by an average of slightly over 1 day while not increasing the risk of death.[51]
At the farming level
It is established that the use of antibiotics in animal husbandry can give rise to AMR resistances in bacteria found in food animals to the antibiotics being administered (through injections or medicated feeds).[52] For this reason only antimicrobials that are deemed "not-clinically relevant" are used in these practices.
Recent studies have shown that the prophylactic use of "non-priority" or "non-clinically relevant" antimicrobials in feeds can potentially, under certain conditions, lead to co-selection of environmental AMR bacteria with resistance to medically important antibiotics.[53] The possibility for co-selection of AMR resistances in the food chain pipeline may have far-reaching implications for human health.[53][54]
At the level of GP
Given the volume of care provided in primary care (General Practice), recent strategies have focused on reducing unnecessary antibiotic prescribing in this setting. Simple interventions, such as written information explaining the futility of antibiotics for common infections such as upper respiratory tract infections, have been shown to reduce antibiotic prescribing.[55]
The prescriber should closely adhere to the five rights of drug administration: the right patient, the right drug, the right dose, the right route, and the right time.[56]
Cultures should be taken before treatment when indicated and treatment potentially changed based on the susceptibility report.[9][57]
About a third of antibiotic prescriptions written in outpatient settings in the United States were not appropriate in 2010 and 2011. Doctors in the U.S. wrote 506 annual antibiotic scripts for every 1,000 people, with 353 being medically necessary.[58]
Health workers and pharmacists can help tackle resistance by: enhancing infection prevention and control; only prescribing and dispensing antibiotics when they are truly needed; prescribing and dispensing the right antibiotic(s) to treat the illness.[24]
At the individual level
People can help tackle resistance by using antibiotics only when prescribed by a doctor; completing the full prescription, even if they feel better; never sharing antibiotics with others or using leftover prescriptions.[24]
Country examples
- The Netherlands has the lowest rate of antibiotic prescribing in the OECD, at a rate of 11.4 defined daily doses (DDD) per 1,000 people per day in 2011.
- Germany and Sweden also have lower prescribing rates, with Sweden's rate having been declining since 2007.
- Greece, France and Belgium have high prescribing rates of more than 28 DDD.[59]
Water, sanitation, hygiene
Infectious disease control through improved water, sanitation and hygiene (WASH) infrastructure needs to be included in the antimicrobial resistance (AMR) agenda. The "Interagency Coordination Group on Antimicrobial Resistance" stated in 2018 that "the spread of pathogens through unsafe water results in a high burden of gastrointestinal disease, increasing even further the need for antibiotic treatment."[60] This is particularly a problem in developing countries where the spread of infectious diseases caused by inadequate WASH standards is a major driver of antibiotic demand.[61] Growing usage of antibiotics together with persistent infectious disease levels have led to a dangerous cycle in which reliance on antimicrobials increases while the efficacy of drugs diminishes.[61] The proper use of infrastructure for water, sanitation and hygiene (WASH) can result in a 47–72 percent decrease of diarrhea cases treated with antibiotics depending on the type of intervention and its effectiveness.[61] A reduction of the diarrhea disease burden through improved infrastructure would result in large decreases in the number of diarrhea cases treated with antibiotics. This was estimated as ranging from 5 million in Brazil to up to 590 million in India by the year 2030.[61] The strong link between increased consumption and resistance indicates that this will directly mitigate the accelerating spread of AMR.[61] Sanitation and water for all by 2030 is Goal Number 6 of the Sustainable Development Goals.
An increase in hand washing compliance by hospital staff results in decreased rates of resistant organisms.[62]
Water supply and sanitation infrastructure in health facilities offer significant co-benefits for combatting AMR, and investment should be increased.[60] There is much room for improvement: WHO and UNICEF estimated in 2015 that globally 38% of health facilities did not have a source of water, nearly 19% had no toilets and 35% had no water and soap or alcohol-based hand rub for handwashing.[63]
Industrial wastewater treatment
Manufacturers of antimicrobials need to improve the treatment of their wastewater (by using industrial wastewater treatment processes) to reduce the release of residues into the environment.[60]
Management in animal use
Europe
In 1997, European Union health ministers voted to ban avoparcin and four additional antibiotics used to promote animal growth in 1999.[64] In 2006 a ban on the use of antibiotics in European feed, with the exception of two antibiotics in poultry feeds, became effective.[65] In Scandinavia, there is evidence that the ban has led to a lower prevalence of antibiotic resistance in (nonhazardous) animal bacterial populations.[66] As of 2004, several European countries established a decline of antimicrobial resistance in humans through limiting the use of antimicrobials in agriculture and food industries without jeopardizing animal health or economic cost.[67]
United States
The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) collect data on antibiotic use in humans and in a more limited fashion in animals.[68] The FDA first determined in 1977 that there is evidence of emergence of antibiotic-resistant bacterial strains in livestock. The long-established practice of permitting OTC sales of antibiotics (including penicillin and other drugs) to lay animal owners for administration to their own animals nonetheless continued in all states. In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter infections in humans. Legal challenges from the food animal and pharmaceutical industries delayed the final decision to do so until 2006.[69] Fluroquinolones have been banned from extra-label use in food animals in the USA since 2007. However, they remain widely used in companion and exotic animals.
Global action plans and awareness
The increasing interconnectedness of the world and the fact that new classes of antibiotics have not been developed and approved for more than 25 years highlight the extent to which antimicrobial resistance is a global health challenge.[70] A global action plan to tackle the growing problem of resistance to antibiotics and other antimicrobial medicines was endorsed at the Sixty-eighth World Health Assembly in May 2015.[71] One of the key objectives of the plan is to improve awareness and understanding of antimicrobial resistance through effective communication, education and training. This global action plan developed by the World Health Organization was created to combat the issue of antimicrobial resistance and was guided by the advice of countries and key stakeholders. The WHO's global action plan is composed of five key objectives that can be targeted through different means, and represents countries coming together to solve a major problem that can have future health consequences.[30] These objectives are as follows:
- improve awareness and understanding of antimicrobial resistance through effective communication, education and training.
- strengthen the knowledge and evidence base through surveillance and research.
- reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures.
- optimize the use of antimicrobial medicines in human and animal health.
- develop the economic case for sustainable investment that takes account of the needs of all countries and to increase investment in new medicines, diagnostic tools, vaccines and other interventions.
Steps towards progress
- React based in Sweden has produced informative material on AMR for the general public.[72]
- Videos are being produced for the general public to generate interest and awareness.[73][74]
- The Irish Department of Health published a National Action Plan on Antimicrobial Resistance in October 2017.[75] The Strategy for the Control of Antimicrobial Resistance in Ireland (SARI), Iaunched in 2001 developed Guidelines for Antimicrobial Stewardship in Hospitals in Ireland[76] in conjunction with the Health Protection Surveillance Centre, these were published in 2009. Following their publication a public information campaign 'Action on Antibiotics[77]' was launched to highlight the need for a change in antibiotic prescribing. Despite this, antibiotic prescribing remains high with variance in adherence to guidelines.[78]
Antibiotic Awareness Week
The World Health Organization has promoted the first World Antibiotic Awareness Week running from 16–22 November 2015. The aim of the week is to increase global awareness of antibiotic resistance. It also wants to promote the correct usage of antibiotics across all fields in order to prevent further instances of antibiotic resistance.[79]
World Antibiotic Awareness Week has been held every November since 2015. For 2017, the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and the World Organisation for Animal Health (OIE) are together calling for responsible use of antibiotics in humans and animals to reduce the emergence of antibiotic resistance.[80]
United Nations
In 2016 the Secretary-General of the United Nations convened the Interagency Coordination Group (IACG) on Antimicrobial Resistance.[81] The IACG worked with international organizations and experts in human, animal, and plant health to create a plan to fight antimicrobial resistance.[81] Their report released in April 2019 highlights the seriousness of antimicrobial resistance and the threat it poses to world health. It suggests five recommendations for member states to follow in order to tackle this increasing threat. The IACG recommendations are as follows:
- Accelerate progress in countries
- Innovate to secure the future
- Collaborate for more effective action
- Invest for a sustainable response
- Strengthen accountability and global governance
Mechanisms and organisms
Bacteria
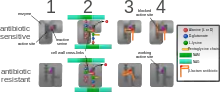
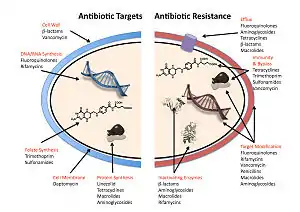
The four main mechanisms by which bacteria exhibit resistance to antibiotics are:
- Drug inactivation or modification: for example, enzymatic deactivation of penicillin G in some penicillin-resistant bacteria through the production of β-lactamases. Most commonly, the protective enzymes produced by the bacterial cell will add an acetyl or phosphate group to a specific site on the antibiotic, which will reduce its ability to bind to the bacterial ribosomes and disrupt protein synthesis.[82]
- Alteration of target- or binding site: for example, alteration of PBP—the binding target site of penicillins—in MRSA and other penicillin-resistant bacteria. Another protective mechanism found among bacterial species is ribosomal protection proteins. These proteins protect the bacterial cell from antibiotics that target the cell's ribosomes to inhibit protein synthesis. The mechanism involves the binding of the ribosomal protection proteins to the ribosomes of the bacterial cell, which in turn changes its conformational shape. This allows the ribosomes to continue synthesizing proteins essential to the cell while preventing antibiotics from binding to the ribosome to inhibit protein synthesis.[83]
- Alteration of metabolic pathway: for example, some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides, instead, like mammalian cells, they turn to using preformed folic acid.[84]
- Reduced drug accumulation: by decreasing drug permeability or increasing active efflux (pumping out) of the drugs across the cell surface[85] These pumps within the cellular membrane of certain bacterial species are used to pump antibiotics out of the cell before they are able to do any damage. They are often activated by a specific substrate associated with an antibiotic.[86] as in fluoroquinolone resistance.[87]
- Ribosome splitting and recycling: for example, drug-mediated stalling of the ribosome by lincomycin and erythromycin unstalled by a heat shock protein found in Listeria monocytogenes, which is a homologue of HflX from other bacteria. Liberation of the ribosome from the drug allows further translation and consequent resistance to the drug.[88]
There are several different types of germs that have developed a resistance over time. For example, Penicillinase-producing Neisseria gonorrhoeae developed a resistance to penicillin in 1976. Another example is Azithromycin-resistant Neisseria gonorrhoeae, which developed a resistance to azithromycin in 2011.[89]
In gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness.[90]
Some bacteria are naturally resistant to certain antibiotics; for example, gram-negative bacteria are resistant to most β-lactam antibiotics due to the presence of β-lactamase. Antibiotic resistance can also be acquired as a result of either genetic mutation or horizontal gene transfer.[91] Although mutations are rare, with spontaneous mutations in the pathogen genome occurring at a rate of about 1 in 105 to 1 in 108 per chromosomal replication,[92] the fact that bacteria reproduce at a high rate allows for the effect to be significant. Given that lifespans and production of new generations can be on a timescale of mere hours, a new (de novo) mutation in a parent cell can quickly become an inherited mutation of widespread prevalence, resulting in the microevolution of a fully resistant colony. However, chromosomal mutations also confer a cost of fitness. For example, a ribosomal mutation may protect a bacterial cell by changing the binding site of an antibiotic but will also slow protein synthesis.[82] manifesting, in slower growth rate.[93] Moreover, some adaptive mutations can propagate not only through inheritance but also through horizontal gene transfer. The most common mechanism of horizontal gene transfer is the transferring of plasmids carrying antibiotic resistance genes between bacteria of the same or different species via conjugation. However, bacteria can also acquire resistance through transformation, as in Streptococcus pneumoniae uptaking of naked fragments of extracellular DNA that contain antibiotic resistance genes to streptomycin,[94] through transduction, as in the bacteriophage-mediated transfer of tetracycline resistance genes between strains of S. pyogenes,[95] or through gene transfer agents, which are particles produced by the host cell that resemble bacteriophage structures and are capable of transferring DNA.[96]
Antibiotic resistance can be introduced artificially into a microorganism through laboratory protocols, sometimes used as a selectable marker to examine the mechanisms of gene transfer or to identify individuals that absorbed a piece of DNA that included the resistance gene and another gene of interest.[97]
Recent findings show no necessity of large populations of bacteria for the appearance of antibiotic resistance. Small populations of Escherichia coli in an antibiotic gradient can become resistant. Any heterogeneous environment with respect to nutrient and antibiotic gradients may facilitate antibiotic resistance in small bacterial populations. Researchers hypothesize that the mechanism of resistance evolution is based on four SNP mutations in the genome of E. coli produced by the gradient of antibiotic.[98]
In one study, which has implications for space microbiology, a non-pathogenic strain E. coli MG1655 was exposed to trace levels of the broad spectrum antibiotic chloramphenicol, under simulated microgravity (LSMMG, or, Low Shear Modeled Microgravity) over 1000 generations. The adapted strain acquired resistance to not only chloramphenicol, but also cross-resistance to other antibiotics;[99] this was in contrast to the observation on the same strain, which was adapted to over 1000 generations under LSMMG, but without any antibiotic exposure; the strain in this case did not acquire any such resistance.[100] Thus, irrespective of where they are used, the use of an antibiotic would likely result in persistent resistance to that antibiotic, as well as cross-resistance to other antimicrobials.
In recent years, the emergence and spread of β-lactamases called carbapenemases has become a major health crisis.[101] One such carbapenemase is New Delhi metallo-beta-lactamase 1 (NDM-1),[102] an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics. The most common bacteria that make this enzyme are gram-negative such as E. coli and Klebsiella pneumoniae, but the gene for NDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.[103]
Viruses
Specific antiviral drugs are used to treat some viral infections. These drugs prevent viruses from reproducing by inhibiting essential stages of the virus's replication cycle in infected cells. Antivirals are used to treat HIV, hepatitis B, hepatitis C, influenza, herpes viruses including varicella zoster virus, cytomegalovirus and Epstein-Barr virus. With each virus, some strains have become resistant to the administered drugs.[104]
Antiviral drugs typically target key components of viral reproduction; for example, oseltamivir targets influenza neuraminidase, while guanosine analogs inhibit viral DNA polymerase. Resistance to antivirals is thus acquired through mutations in the genes that encode the protein targets of the drugs.
Resistance to HIV antivirals is problematic, and even multi-drug resistant strains have evolved.[105] One source of resistance is that many current HIV drugs, including NRTIs and NNRTIs, target reverse transcriptase; however, HIV-1 reverse transcriptase is highly error prone and thus mutations conferring resistance arise rapidly.[106] Resistant strains of the HIV virus emerge rapidly if only one antiviral drug is used.[107] Using three or more drugs together, termed combination therapy, has helped to control this problem, but new drugs are needed because of the continuing emergence of drug-resistant HIV strains.[108]
Fungi
Infections by fungi are a cause of high morbidity and mortality in immunocompromised persons, such as those with HIV/AIDS, tuberculosis or receiving chemotherapy.[109] The fungi candida, Cryptococcus neoformans and Aspergillus fumigatus cause most of these infections and antifungal resistance occurs in all of them.[110] Multidrug resistance in fungi is increasing because of the widespread use of antifungal drugs to treat infections in immunocompromised individuals.[111]
Of particular note, Fluconazole-resistant Candida species have been highlighted as a growing problem by the CDC.[41] More than 20 species of Candida can cause Candidiasis infection, the most common of which is Candida albicans. Candida yeasts normally inhabit the skin and mucous membranes without causing infection. However, overgrowth of Candida can lead to Candidiasis. Some Candida strains are becoming resistant to first-line and second-line antifungal agents such as azoles and echinocandins.[41]
Parasites
The protozoan parasites that cause the diseases malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis are important human pathogens.[112]
Malarial parasites that are resistant to the drugs that are currently available to infections are common and this has led to increased efforts to develop new drugs.[113] Resistance to recently developed drugs such as artemisinin has also been reported. The problem of drug resistance in malaria has driven efforts to develop vaccines.[114]
Trypanosomes are parasitic protozoa that cause African trypanosomiasis and Chagas disease (American trypanosomiasis).[115][116] There are no vaccines to prevent these infections so drugs such as pentamidine and suramin, benznidazole and nifurtimox are used to treat infections. These drugs are effective but infections caused by resistant parasites have been reported.[112]
Leishmaniasis is caused by protozoa and is an important public health problem worldwide, especially in sub-tropical and tropical countries. Drug resistance has "become a major concern".[117]
History
The 1950s to 1970s represented the golden age of antibiotic discovery, where countless new classes of antibiotics were discovered to treat previously incurable diseases such as tuberculosis and syphilis.[118] However, since that time the discovery of new classes of antibiotics has been almost nonexistent, and represents a situation that is especially problematic considering the resiliency of bacteria[119] shown over time and the continued misuse and overuse of antibiotics in treatment.[120]
The phenomenon of antimicrobial resistance caused by overuse of antibiotics was predicted as early as 1945 by Alexander Fleming who said "The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily under-dose himself and by exposing his microbes to nonlethal quantities of the drug make them resistant."[121][122] Without the creation of new and stronger antibiotics an era where common infections and minor injuries can kill, and where complex procedures such as surgery and chemotherapy become too risky, is a very real possibility.[123] Antimicrobial resistance threatens the world as we know it, and can lead to epidemics of enormous proportions if preventive actions are not taken. In this day and age current antimicrobial resistance leads to longer hospital stays, higher medical costs, and increased mortality.[120]
Society and culture
Since the mid-1980s pharmaceutical companies have invested in medications for cancer or chronic disease that have greater potential to make money and have "de-emphasized or dropped development of antibiotics".[124] On 20 January 2016 at the World Economic Forum in Davos, Switzerland, more than "80 pharmaceutical and diagnostic companies" from around the world called for "transformational commercial models" at a global level to spur research and development on antibiotics and on the "enhanced use of diagnostic tests that can rapidly identify the infecting organism".[124]
Legal frameworks
Some global health scholars have argued that a global, legal framework is needed to prevent and control antimicrobial resistance.[125][126][20][127] For instance, binding global policies could be used to create antimicrobial use standards, regulate antibiotic marketing, and strengthen global surveillance systems.[20][125] Ensuring compliance of involved parties is a challenge.[20] Global antimicrobial resistance policies could take lessons from the environmental sector by adopting strategies that have made international environmental agreements successful in the past such as: sanctions for non-compliance, assistance for implementation, majority vote decision-making rules, an independent scientific panel, and specific commitments.[128]
United States
For the United States 2016 budget, U.S. president Barack Obama proposed to nearly double the amount of federal funding to "combat and prevent" antibiotic resistance to more than $1.2 billion.[129] Many international funding agencies like USAID, DFID, SIDA and Bill & Melinda Gates Foundation have pledged money for developing strategies to counter antimicrobial resistance.
On 27 March 2015, the White House released a comprehensive plan to address the increasing need for agencies to combat the rise of antibiotic-resistant bacteria. The Task Force for Combating Antibiotic-Resistant Bacteria developed The National Action Plan for Combating Antibiotic-Resistant Bacteria with the intent of providing a roadmap to guide the US in the antibiotic resistance challenge and with hopes of saving many lives. This plan outlines steps taken by the Federal government over the next five years needed in order to prevent and contain outbreaks of antibiotic-resistant infections; maintain the efficacy of antibiotics already on the market; and to help to develop future diagnostics, antibiotics, and vaccines.[130]
The Action Plan was developed around five goals with focuses on strengthening health care, public health veterinary medicine, agriculture, food safety and research, and manufacturing. These goals, as listed by the White House, are as follows:
- Slow the Emergence of Resistant Bacteria and Prevent the Spread of Resistant Infections
- Strengthen National One-Health Surveillance Efforts to Combat Resistance
- Advance Development and use of Rapid and Innovative Diagnostic Tests for Identification and Characterization of Resistant Bacteria
- Accelerate Basic and Applied Research and Development for New Antibiotics, Other Therapeutics, and Vaccines
- Improve International Collaboration and Capacities for Antibiotic Resistance Prevention, Surveillance, Control and Antibiotic Research and Development
The following are goals set to meet by 2020:[130]
- Establishment of antimicrobial programs within acute care hospital settings
- Reduction of inappropriate antibiotic prescription and use by at least 50% in outpatient settings and 20% inpatient settings
- Establishment of State Antibiotic Resistance (AR) Prevention Programs in all 50 states
- Elimination of the use of medically important antibiotics for growth promotion in food-producing animals.
United Kingdom
Public Health England reported that the total number of antibiotic resistant infections in England rose by 9% from 55,812 in 2017 to 60,788 in 2018, but antibiotic consumption had fallen by 9% from 20.0 to 18.2 defined daily doses per 1,000 inhabitants per day between 2014 and 2018.[131]
Policies
According to World Health Organization, policymakers can help tackle resistance by strengthening resistance-tracking and laboratory capacity and by regulating and promoting the appropriate use of medicines.[24] Policymakers and industry can help tackle resistance by: fostering innovation and research and development of new tools; and promoting cooperation and information sharing among all stakeholders.[24]
Further research
Rapid viral testing
Clinical investigation to rule out bacterial infections are often done for patients with pediatric acute respiratory infections. Currently it is unclear if rapid viral testing affects antibiotic use in children.[132]
Vaccines
Microorganisms do not develop resistance to vaccines because a vaccine enhances the body's immune system, whereas an antibiotic operates separately from the body's normal defenses. Furthermore, if the use of vaccines increases, there is evidence that antibiotic resistant strains of pathogens will decrease; the need for antibiotics will naturally decrease as vaccines prevent infection before it occurs.[133] However, new strains that escape immunity induced by vaccines may evolve; for example, an updated influenza vaccine is needed each year.
While theoretically promising, antistaphylococcal vaccines have shown limited efficacy, because of immunological variation between Staphylococcus species, and the limited duration of effectiveness of the antibodies produced. Development and testing of more effective vaccines is underway.[134]
Two registrational trials have evaluated vaccine candidates in active immnization sttrategies against S. aureus infection. In a phase II trial, a bivalent vaccine of capsulat prtein 5 & 8 was tested in 1804 hemodialysis patients with a primary fistula or synthetic graft vascular access. After 40 weeks following vaccination a protective effect was seen against S. aureus bacteremia, but not at 54 weeks following vaccination.[135] Based on these results, a second trial was conducted which failed to show efficacy.[136]
Merck tested V710, a vaccine targeting IsdB, in a blinded randomized trial in patients undergoing median sternotomy. The trial was terminated after a higher rate of multiorgan system failure–related deaths was found in the V710 recipients. Vaccine recipients who developed S. aureus infection were 5 times more likely to die than control recipients who developed S. aureus infection.[137]
Numerous investigators have suggested that a multiple-antigen vaccine would be more effective, but a lack of biomarkers defining human protective immunity keep these proposals in the logical, but strictly hypothetical arena.[136]
Alternating therapy
Alternating therapy is a proposed method in which two or three antibiotics are taken in a rotation versus taking just one antibiotic such that bacteria resistant to one antibiotic are killed when the next antibiotic is taken. Studies have found that this method reduces the rate at which antibiotic resistant bacteria emerge in vitro relative to a single drug for the entire duration.[138]
Studies have found that bacteria that evolve antibiotic resistance towards one group of antibiotic may become more sensitive to others.[139] This phenomenon can be used to select against resistant bacteria using an approach termed collateral sensitivity cycling,[140] which has recently been found to be relevant in developing treatment strategies for chronic infections caused by Pseudomonas aeruginosa.[141]
Development of new drugs
Since the discovery of antibiotics, research and development (R&D) efforts have provided new drugs in time to treat bacteria that became resistant to older antibiotics, but in the 2000s there has been concern that development has slowed enough that seriously ill people may run out of treatment options.[142][143] Another concern is that doctors may become reluctant to perform routine surgeries because of the increased risk of harmful infection.[144] Backup treatments can have serious side-effects; for example, treatment of multi-drug-resistant tuberculosis can cause deafness or psychological disability.[145] The potential crisis at hand is the result of a marked decrease in industry R&D.[146] Poor financial investment in antibiotic research has exacerbated the situation.[147][146] The pharmaceutical industry has little incentive to invest in antibiotics because of the high risk and because the potential financial returns are less likely to cover the cost of development than for other pharmaceuticals.[148] In 2011, Pfizer, one of the last major pharmaceutical companies developing new antibiotics, shut down its primary research effort, citing poor shareholder returns relative to drugs for chronic illnesses.[149] However, small and medium-sized pharmaceutical companies are still active in antibiotic drug research.
In the United States, drug companies and the administration of President Barack Obama had been proposing changing the standards by which the FDA approves antibiotics targeted at resistant organisms.[144][150]
On 18 September 2014 Obama signed an executive order[151] to implement the recommendations proposed in a report[152] by the President's Council of Advisors on Science and Technology (PCAST) which outlines strategies to stream-line clinical trials and speed up the R&D of new antibiotics. Among the proposals:
- Create a 'robust, standing national clinical trials network for antibiotic testing' which will promptly enroll patients once identified to be suffering from dangerous bacterial infections. The network will allow testing multiple new agents from different companies simultaneously for their safety and efficacy.
- Establish a 'Special Medical Use (SMU)' pathway for FDA to approve new antimicrobial agents for use in limited patient populations, shorten the approval timeline for new drug so patients with severe infections could benefit as quickly as possible.
- Provide economic incentives, especially for development of new classes of antibiotics, to offset the steep R&D costs which drive away the industry to develop antibiotics.
Biomaterials
Using antibiotic-free alternatives in bone infection treatment may help decrease the use of antibiotics and thus antimicrobial resistance.[153] The bone regeneration material bioactive glass S53P4 has shown to effectively inhibit the bacterial growth of up to 50 clinically relevant bacteria including MRSA and MRSE.[154][155][156]
Rediscovery of ancient treatments
Similar to the situation in malaria therapy, where successful treatments based on ancient recipes have been found,[157] there has already been some success in finding and testing ancient drugs and other treatments that are effective against AMR bacteria.[158]
Rapid diagnostics

Distinguishing infections requiring antibiotics from self-limiting ones is clinically challenging. In order to guide appropriate use of antibiotics and prevent the evolution and spread of antimicrobial resistance, diagnostic tests that provide clinicians with timely, actionable results are needed.
Acute febrile illness is a common reason for seeking medical care worldwide and a major cause of morbidity and mortality. In areas with decreasing malaria incidence, many febrile patients are inappropriately treated for malaria, and in the absence of a simple diagnostic test to identify alternative causes of fever, clinicians presume that a non-malarial febrile illness is most likely a bacterial infection, leading to inappropriate use of antibiotics. Multiple studies have shown that the use of malaria rapid diagnostic tests without reliable tools to distinguish other fever causes has resulted in increased antibiotic use.[159]
Antimicrobial susceptibility testing (AST) can help practitioners avoid prescribing unnecessary antibiotics in the style of precision medicine,[160] and help them prescribe effective antibiotics, but with the traditional approach it could take 12 to 48 hours.[161] Rapid testing, possible from molecular diagnostics innovations, is defined as "being feasible within an 8-h working shift".[161] Progress has been slow due to a range of reasons including cost and regulation.[162]
Phage therapy
Phage therapy is the therapeutic use of bacteriophages to treat pathogenic bacterial infections.[163] Phage therapy has many potential applications in human medicine as well as dentistry, veterinary science, and agriculture.[164]
Phage therapy relies on the use of naturally-occurring bacteriophages to infect and lyse bacteria at the site of infection in a host. Due to current advances in genetics and biotechnology these bacteriophages can possibly be manufactured to treat specific infections.[165] Phages can be bioengineered to target multidrug-resistant bacterial infections, and their use involves the added benefit of preventing the elimination of beneficial bacteria in the human body.[31] Phages destroy bacterial cell walls and membrane through the use of lytic proteins which kill bacteria by making many holes from the inside out.[166] Bacteriophages can even possess the ability to digest the biofilm that many bacteria develop that protect them from antibiotics in order to effectively infect and kill bacteria. Bioengineering can play a role in creating successful bacteriophages.[166]
Understanding the mutual interactions and evolutions of bacterial and phage populations in the environment of a human or animal body is essential for rational phage therapy.[167]
Bacteriophagics are used against antibiotic resistant bacteria in Georgia (George Eliava Institute) and in one institute in Wrocław, Poland.[168][169] Bacteriophage cocktails are common drugs sold over the counter in pharmacies in eastern countries.[170][171] In Belgium, four patients with severe musckuloskeletal infections received bacteriophage therapy with concomitant antibiotics. After a single course of phage therapy, no recurrence of infection occurred and no severe side-effects related to the therapy were detected.[172]
See also
- Alliance for the Prudent Use of Antibiotics
- Broad-spectrum antibiotic
- Colonisation resistance
- Drug of last resort
- Genetic engineering
- (KPC) antibacterial resistance gene
- Multidrug-resistant Gram-negative bacteria
- New Delhi metallo-beta-lactamase 1
- Persister cells
- Resistance-nodulation-cell division superfamily (RND)
- Resistome
References
- Kirby-Bauer Disk Diffusion Susceptibility Test Protocol Archived 26 June 2011 at the Wayback Machine, Jan Hudzicki, ASM
- "Antimicrobial resistance Fact sheet N°194". who.int. April 2014. Archived from the original on 10 March 2015. Retrieved 7 March 2015.
- A.-P. Magiorakos, A. Srinivasan, R. B. Carey, Y. Carmeli, M. E. Falagas, C. G. Giske, S. Harbarth, J. F. Hinndler et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria.... Clinical Microbiology and Infection, Vol 8, Iss. 3 first published 27 July 2011 [via Wiley Online Library]. Retrieved 28 August 2020
- "General Background: About Antibiotic Resistance". www.tufts.edu. Archived from the original on 23 October 2015. Retrieved 30 October 2015.
- "About Antimicrobial Resistance". www.cdc.gov. 10 September 2018. Archived from the original on 1 October 2017. Retrieved 30 October 2015.
- Swedish work on containment of antibiotic resistance – Tools, methods and experiences (PDF). Stockholm: Public Health Agency of Sweden. 2014. pp. 16–17, 121–128. ISBN 978-91-7603-011-0. Archived (PDF) from the original on 23 July 2015. Retrieved 23 July 2015.
- "Duration of antibiotic therapy and resistance". NPS Medicinewise. National Prescribing Service Limited trading, Australia. 13 June 2013. Archived from the original on 23 July 2015. Retrieved 22 July 2015.
- Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, et al. (December 2017). "Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections". JAMA. 318 (23): 2325–2336. doi:10.1001/jama.2017.18715. PMC 5820700. PMID 29260224.
- "CDC Features – Mission Critical: Preventing Antibiotic Resistance". www.cdc.gov. 4 April 2018. Archived from the original on 8 November 2017. Retrieved 22 July 2015.
- Changing Markets. "IMPACTS OF PHARMACEUTICAL POLLUTION ON COMMUNITIES AND ENVIRONMENT IN INDIA" (PDF). Nordea. Nordea. Archived (PDF) from the original on 20 May 2017. Retrieved 1 May 2018.
- Gullberg E, Cao S, Berg OG, Ilbäck C, Sandegren L, Hughes D, Andersson DI (July 2011). "Selection of resistant bacteria at very low antibiotic concentrations". PLOS Pathogens. 7 (7): e1002158. doi:10.1371/journal.ppat.1002158. PMC 3141051. PMID 21811410.
- Cassir N, Rolain JM, Brouqui P (2014). "A new strategy to fight antimicrobial resistance: the revival of old antibiotics". Frontiers in Microbiology. 5: 551. doi:10.3389/fmicb.2014.00551. PMC 4202707. PMID 25368610.
- Sample I (26 March 2018). "Calls to rein in antibiotic use after study shows 65% increase worldwide". The Guardian. Archived from the original on 8 April 2018. Retrieved 28 March 2018.
- Drame, O., Leclair, D., Parmley, E. J., et al Antimicrobial resistance of Campylobacter in broiler chicken along the food chain in Canada. Foodborne Pathogens and Disease; 2020;17(8):512-520. doi:10.1089/fpd.2019.2752
- WHO (April 2014). "Antimicrobial resistance: global report on surveillance 2014". WHO. WHO. Archived from the original on 15 May 2015. Retrieved 9 May 2015.
- O'Neill J (May 2016). "Tackling drug-resistant infections globally: final report and recommendations" (PDF). amr-review.org/. Archived (PDF) from the original on 14 November 2017. Retrieved 10 November 2017.
- "The biggest antibiotic-resistant threats in the U.S." Centers for Disease Control and Prevention. 6 November 2019. Retrieved 15 November 2019.
- Chanel, Sheldon; Doherty, Ben (10 September 2020). "'Superbugs' a far greater risk than Covid in Pacific, scientist warns". The Guardian. ISSN 0261-3077. Retrieved 14 September 2020.
- Samuel, Sigal (7 May 2019). "Our antibiotics are becoming useless". Vox. Retrieved 28 January 2021.
- Hoffman SJ, Outterson K, Røttingen JA, Cars O, Clift C, Rizvi Z, et al. (February 2015). "An international legal framework to address antimicrobial resistance". Bulletin of the World Health Organization. 93 (2): 66. doi:10.2471/BLT.15.152710. PMC 4339972. PMID 25883395.
- "What is Drug Resistance?". www.niaid.nih.gov. Archived from the original on 27 July 2015. Retrieved 26 July 2015.
- "CDC: Get Smart: Know When Antibiotics Work". Cdc.gov. 29 May 2018. Archived from the original on 29 April 2015. Retrieved 12 June 2013.
- MacGowan A, Macnaughton E (1 October 2017). "Antibiotic resistance". Medicine. 45 (10): 622–628. doi:10.1016/j.mpmed.2017.07.006.
- "WHO's first global report on antibiotic resistance reveals serious, worldwide threat to public health" Archived 2 May 2014 at the Wayback Machine Retrieved 2 May 2014
- "Antibiotic resistance". www.who.int. Retrieved 16 March 2020.
- "Antibiotic-resistant bacteria responsible for over 33,000 deaths in Europe in 2015, study finds". Pharmaceutical Journal. 7 November 2018. Retrieved 16 December 2018.
- "Antimicrobial Resistance " Cambridge Medicine Journal". Retrieved 27 February 2020.
- Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. (January 2016). "Understanding the mechanisms and drivers of antimicrobial resistance". Lancet. 387 (10014): 176–87. doi:10.1016/S0140-6736(15)00473-0. hdl:10044/1/32225. PMID 26603922. S2CID 1944665.
- "Natural selection". evolution.berkeley.edu. Retrieved 10 March 2020.
- Ferri M, Ranucci E, Romagnoli P, Giaccone V (September 2017). "Antimicrobial resistance: A global emerging threat to public health systems". Critical Reviews in Food Science and Nutrition. 57 (13): 2857–2876. doi:10.1080/10408398.2015.1077192. PMID 26464037. S2CID 24549694.
- Rather IA, Kim BC, Bajpai VK, Park YH (May 2017). "Self-medication and antibiotic resistance: Crisis, current challenges, and prevention". Saudi Journal of Biological Sciences. 24 (4): 808–812. doi:10.1016/j.sjbs.2017.01.004. PMC 5415144. PMID 28490950.
- Ayukekbong JA, Ntemgwa M, Atabe AN (15 May 2017). "The threat of antimicrobial resistance in developing countries: causes and control strategies". Antimicrobial Resistance and Infection Control. 6 (1): 47. doi:10.1186/s13756-017-0208-x. PMC 5433038. PMID 28515903.
- Ventola CL (April 2015). "The antibiotic resistance crisis: part 1: causes and threats". P & T. 40 (4): 277–83. PMC 4378521. PMID 25859123.
- Strachan, Cameron R.; Davies, Julian (1 February 2017). "The Whys and Wherefores of Antibiotic Resistance". Cold Spring Harbor Perspectives in Medicine. 7 (2): a025171. doi:10.1101/cshperspect.a025171. ISSN 2157-1422. PMC 5287056. PMID 27793964.
- Harris A, Chandramohan S, Awali RA, Grewal M, Tillotson G, Chopra T (August 2019). "Physicians' attitude and knowledge regarding antibiotic use and resistance in ambulatory settings". American Journal of Infection Control. 47 (8): 864–868. doi:10.1016/j.ajic.2019.02.009. PMID 30926215.
- Ahmad, Akram (June 2017). "Pharmaceutical waste and antimicrobial resistance". Lancet. 17 (6): 578–579. doi:10.1016/S1473-3099(17)30268-2. PMID 28555576. Retrieved 7 October 2020.
- Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, et al. (November 2017). "Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis". The Lancet. Planetary Health. 1 (8): e316–e327. doi:10.1016/S2542-5196(17)30141-9. PMC 5785333. PMID 29387833.
- Innes GK, Randad PR, Korinek A, Davis MF, Price LB, So AD, Heaney CD (April 2020). "External Societal Costs of Antimicrobial Resistance in Humans Attributable to Antimicrobial Use in Livestock". Annual Review of Public Health. 41 (1): 141–157. doi:10.1146/annurev-publhealth-040218-043954. PMC 7199423. PMID 31910712.
- US EPA, OCSPP (15 March 2013). "What are Antimicrobial Pesticides?". US EPA. Retrieved 28 February 2020.
- Ramakrishnan B, Venkateswarlu K, Sethunathan N, Megharaj M (March 2019). "Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance?". The Science of the Total Environment. 654: 177–189. Bibcode:2019ScTEn.654..177R. doi:10.1016/j.scitotenv.2018.11.041. PMID 30445319.
- "Biggest Threats – Antibiotic/Antimicrobial Resistance – CDC". www.cdc.gov. 10 September 2018. Archived from the original on 12 September 2017. Retrieved 5 May 2016.
- "HealthMap Resistance". HealthMap.org Boston Children's Hospital. Archived from the original on 15 November 2017. Retrieved 15 November 2017.
- Scales D. "Mapping Antibiotic Resistance: Know The Germs in Your Neighborhood". WBUR. National Public Radio. Archived from the original on 8 December 2015. Retrieved 8 December 2015.
- "ResistanceMap". Center for Disease Dynamics, Economics & Policy. Archived from the original on 14 November 2017. Retrieved 14 November 2017.
- Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele S, Tacconelli E (September 2017). "Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 17 (9): 990–1001. doi:10.1016/S1473-3099(17)30325-0. PMID 28629876.
- Gallagher JC, Justo JA, Chahine EB, Bookstaver PB, Scheetz M, Suda KJ, et al. (August 2018). "Preventing the Post-Antibiotic Era by Training Future Pharmacists as Antimicrobial Stewards". American Journal of Pharmaceutical Education. 82 (6): 6770. doi:10.5688/ajpe6770. PMC 6116871. PMID 30181677.
- Andersson DI, Hughes D (September 2011). "Persistence of antibiotic resistance in bacterial populations". FEMS Microbiology Reviews. 35 (5): 901–11. doi:10.1111/j.1574-6976.2011.00289.x. PMID 21707669.
- Gilberg K, Laouri M, Wade S, Isonaka S (2003). "Analysis of medication use patterns:apparent overuse of antibiotics and underuse of prescription drugs for asthma, depression, and CHF". Journal of Managed Care Pharmacy. 9 (3): 232–7. doi:10.18553/jmcp.2003.9.3.232. PMID 14613466. S2CID 25457069.
- Llor C, Bjerrum L (December 2014). "Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem". Therapeutic Advances in Drug Safety. 5 (6): 229–41. doi:10.1177/2042098614554919. PMC 4232501. PMID 25436105.
- Doron S, Davidson LE (November 2011). "Antimicrobial stewardship". Mayo Clinic Proceedings. 86 (11): 1113–23. doi:10.4065/mcp.2011.0358. PMC 3203003. PMID 22033257.
- Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. (February 2017). "Interventions to improve antibiotic prescribing practices for hospital inpatients". The Cochrane Database of Systematic Reviews. 2: CD003543. doi:10.1002/14651858.cd003543.pub4. PMC 6464541. PMID 28178770.
- Agga GE, Schmidt JW, Arthur TM (December 2016). "Effects of In-Feed Chlortetracycline Prophylaxis in Beef Cattle on Animal Health and Antimicrobial-Resistant Escherichia coli". Applied and Environmental Microbiology. 82 (24): 7197–7204. doi:10.1128/AEM.01928-16. PMC 5118930. PMID 27736789.
- Brown EE, Cooper A, Carrillo C, Blais B (2019). "Selection of Multidrug-Resistant Bacteria in Medicated Animal Feeds". Frontiers in Microbiology. 10: 456. doi:10.3389/fmicb.2019.00456. PMC 6414793. PMID 30894847.
- Marshall BM, Levy SB (October 2011). "Food animals and antimicrobials: impacts on human health". Clinical Microbiology Reviews. 24 (4): 718–33. doi:10.1128/CMR.00002-11. PMC 3194830. PMID 21976606.
- O'Sullivan JW, Harvey RT, Glasziou PP, McCullough A (November 2016). "Written information for patients (or parents of child patients) to reduce the use of antibiotics for acute upper respiratory tract infections in primary care". The Cochrane Database of Systematic Reviews. 11: CD011360. doi:10.1002/14651858.CD011360.pub2. PMC 6464519. PMID 27886368.
- "The Five Rights of Medication Administration". www.ihi.org. Archived from the original on 24 October 2015. Retrieved 30 October 2015.
- Leekha S, Terrell CL, Edson RS (February 2011). "General principles of antimicrobial therapy". Mayo Clinic Proceedings. 86 (2): 156–67. doi:10.4065/mcp.2010.0639. PMC 3031442. PMID 21282489.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, et al. (May 2016). "Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011". JAMA. 315 (17): 1864–73. doi:10.1001/jama.2016.4151. PMID 27139059.
- "Indicator: Antibiotic prescribing". QualityWatch. Nuffield Trust & Health Foundation. Archived from the original on 14 January 2015. Retrieved 16 July 2015.
- IACG (2018) Reduce unintentional exposure and the need for antimicrobials, and optimize their use IACG Discussion Paper, Interagency Coordination Group on Antimicrobial Resistance, public consultation process at WHO, Geneva, Switzerland
- Araya P (May 2016). "The Impact of Water and Sanitation on Diarrhoeal Disease Burden and Over-Consumption of Anitbiotics" (PDF). Archived (PDF) from the original on 1 October 2017. Retrieved 12 November 2017.
- Swoboda SM, Earsing K, Strauss K, Lane S, Lipsett PA (February 2004). "Electronic monitoring and voice prompts improve hand hygiene and decrease nosocomial infections in an intermediate care unit". Critical Care Medicine. 32 (2): 358–63. doi:10.1097/01.CCM.0000108866.48795.0F. PMID 14758148. S2CID 9817602.(subscription required)
- WHO, UNICEF (2015). Water, sanitation and hygiene in health care facilities – Status in low and middle income countries and way forward Archived 12 September 2018 at the Wayback Machine. World Health Organization (WHO), Geneva, Switzerland, ISBN 978 92 4 150847 6
- Casewell M, Friis C, Marco E, McMullin P, Phillips I (August 2003). "The European ban on growth-promoting antibiotics and emerging consequences for human and animal health". The Journal of Antimicrobial Chemotherapy. 52 (2): 159–61. doi:10.1093/jac/dkg313. PMID 12837737.
- Castanon JI (November 2007). "History of the use of antibiotic as growth promoters in European poultry feeds". Poultry Science. 86 (11): 2466–71. doi:10.3382/ps.2007-00249. PMID 17954599.(subscription required)
- Bengtsson B, Wierup M (2006). "Antimicrobial resistance in Scandinavia after ban of antimicrobial growth promoters". Animal Biotechnology. 17 (2): 147–56. doi:10.1080/10495390600956920. PMID 17127526. S2CID 34602891.(subscription required)
- Angulo FJ, Baker NL, Olsen SJ, Anderson A, Barrett TJ (April 2004). "Antimicrobial use in agriculture: controlling the transfer of antimicrobial resistance to humans". Seminars in Pediatric Infectious Diseases. 15 (2): 78–85. doi:10.1053/j.spid.2004.01.010. PMID 15185190.
- "GAO-11-801, Antibiotic Resistance: Agencies Have Made Limited Progress Addressing Antibiotic Use in Animals". gao.gov. Archived from the original on 5 November 2013. Retrieved 25 January 2014.
- Nelson JM, Chiller TM, Powers JH, Angulo FJ (April 2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story". Clinical Infectious Diseases. 44 (7): 977–80. doi:10.1086/512369. PMID 17342653.
- "RAND Europe Focus on Antimicrobial Resistance (AMR)". www.rand.org. Archived from the original on 21 April 2018. Retrieved 23 April 2018.
- WHO. "GLOBAL ACTION PLAN ON ANTIMICROBIAL RESISTANCE" (PDF). Archived (PDF) from the original on 31 October 2017. Retrieved 14 November 2017.
- "React". Archived from the original on 16 November 2017. Retrieved 16 November 2017.
- "Antibiotic Resistance: the silent tsunami (youtube video)". ReActTube. 6 March 2017. Retrieved 17 November 2017.
- "The Antibiotic Apocalypse Explained". Kurzgesagt – In a Nutshell. 16 March 2016. Retrieved 17 November 2017.
- Health (DoH), Department of (October 2017). "Ireland's National Action Plan on Antimicrobial Resistance 2017 – 2020" – via Lenus (Irish Health Repository).
- Group, SARI Hospital Antimicrobial Stewardship Working (2009). Guidelines for antimicrobial stewardship in hospitals in Ireland. Dublin: HSE Health Protection Surveillance Centre (HPSC). ISBN 9780955123672.
- "Taking antibiotics for colds and flu? There's no point". HSE.ie. Retrieved 11 January 2019.
- Murphy M, Bradley CP, Byrne S (May 2012). "Antibiotic prescribing in primary care, adherence to guidelines and unnecessary prescribing--an Irish perspective". BMC Family Practice. 13: 43. doi:10.1186/1471-2296-13-43. PMC 3430589. PMID 22640399.
- "World Antibiotic Awareness Week". World Health Organization. Archived from the original on 20 November 2015. Retrieved 20 November 2015.
- "World Antibiotic Awareness Week". WHO. Archived from the original on 13 November 2017. Retrieved 14 November 2017.
- "WHO | UN Interagency Coordination Group (IACG) on Antimicrobial Resistance". WHO. Retrieved 7 August 2019.
- [Criswell, Daniel. "The "Evolution" of Antibiotic Resistance." Institute for Creation Research. N.p., 2004. Web. 28 October 2014.]
- Connell SR, Tracz DM, Nierhaus KH, Taylor DE (December 2003). "Ribosomal protection proteins and their mechanism of tetracycline resistance". Antimicrobial Agents and Chemotherapy. 47 (12): 3675–81. doi:10.1128/AAC.47.12.3675-3681.2003. PMC 296194. PMID 14638464.
- Henry RJ (December 1943). "The Mode of Action of Sulfonamides". Bacteriological Reviews. 7 (4): 175–262. doi:10.1128/MMBR.7.4.175-262.1943. PMC 440870. PMID 16350088.
- Li XZ, Nikaido H (August 2009). "Efflux-mediated drug resistance in bacteria: an update". Drugs. 69 (12): 1555–623. doi:10.2165/11317030-000000000-00000. PMC 2847397. PMID 19678712.
- Aminov RI, Mackie RI (June 2007). "Evolution and ecology of antibiotic resistance genes". FEMS Microbiology Letters. 271 (2): 147–61. doi:10.1111/j.1574-6968.2007.00757.x. PMID 17490428.
- Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T (July 1998). "NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli". Antimicrobial Agents and Chemotherapy. 42 (7): 1778–82. doi:10.1128/AAC.42.7.1778. PMC 105682. PMID 9661020.
- Duval M, Dar D, Carvalho F, Rocha EP, Sorek R, Cossart P (December 2018). "HflXr, a homolog of a ribosome-splitting factor, mediates antibiotic resistance". Proceedings of the National Academy of Sciences of the United States of America. 115 (52): 13359–13364. doi:10.1073/pnas.1810555115. PMC 6310831. PMID 30545912.
- "About Antibiotic Resistance". CDC.
- Robicsek A, Jacoby GA, Hooper DC (October 2006). "The worldwide emergence of plasmid-mediated quinolone resistance". The Lancet. Infectious Diseases. 6 (10): 629–40. doi:10.1016/S1473-3099(06)70599-0. PMID 17008172.
- Ochiai K, Yamanaka T, Kimura K, Sawada O, O (1959). "Inheritance of drug resistance (and its transfer) between Shigella strains and Between Shigella and E.coli strains". Hihon Iji Shimpor (in Japanese). 34: 1861.
- Watford S, Warrington SJ (2018), "Bacterial DNA Mutations", StatPearls, StatPearls Publishing, PMID 29083710, retrieved 21 January 2019
- Levin BR, Perrot V, Walker N (March 2000). "Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria". Genetics. 154 (3): 985–97. PMC 1460977. PMID 10757748.
- Hotchkiss RD (1951). "Transfer of penicillin resistance in pneumococci by the desoxyribonucleate derived from resistant cultures". Cold Spring Harbor Symposia on Quantitative Biology. 16: 457–61. doi:10.1101/SQB.1951.016.01.032. PMID 14942755.
- Ubukata K, Konno M, Fujii R (September 1975). "Transduction of drug resistance to tetracycline, chloramphenicol, macrolides, lincomycin and clindamycin with phages induced from Streptococcus pyogenes". The Journal of Antibiotics. 28 (9): 681–8. doi:10.7164/antibiotics.28.681. PMID 1102514.
- von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, et al. (19 February 2016). "Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer". Frontiers in Microbiology. 7: 173. doi:10.3389/fmicb.2016.00173. PMC 4759269. PMID 26925045.
- Chan CX, Beiko RG, Ragan MA (August 2011). "Lateral transfer of genes and gene fragments in Staphylococcus extends beyond mobile elements". Journal of Bacteriology. 193 (15): 3964–77. doi:10.1128/JB.01524-10. PMC 3147504. PMID 21622749.
- Johansen TB, Scheffer L, Jensen VK, Bohlin J, Feruglio SL (June 2018). "Whole-genome sequencing and antimicrobial resistance in Brucella melitensis from a Norwegian perspective". Scientific Reports. 8 (1): 8538. Bibcode:2018NatSR...8.8538J. doi:10.1038/s41598-018-26906-3. PMC 5986768. PMID 29867163.
- Tirumalai MR, Karouia F, Tran Q, Stepanov VG, Bruce RJ, Ott M, Pierson DL, Fox GE (January 2019). "Evaluation of acquired antibiotic resistance in Escherichia coli exposed to long-term low-shear modeled microgravity and background antibiotic exposure". mBio. 10 (e02637-18). doi:10.1128/mBio.02637-18. PMC 6336426. PMID 30647159.
- Tirumalai MR, Karouia F, Tran Q, Stepanov VG, Bruce RJ, Ott M, Pierson DL, Fox GE (May 2017). "The adaptation of Escherichia coli cells grown in simulated microgravity for an extended period is both phenotypic and genomic". NPJ Microgravity. 3 (15): 15. doi:10.1038/s41526-017-0020-1. PMC 5460176. PMID 28649637.
- Diene SM, Rolain JM (September 2014). "Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species". Clinical Microbiology and Infection. 20 (9): 831–8. doi:10.1111/1469-0691.12655. PMID 24766097.
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. (September 2010). "Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study". The Lancet. Infectious Diseases. 10 (9): 597–602. doi:10.1016/S1473-3099(10)70143-2. PMC 2933358. PMID 20705517.
- Hudson CM, Bent ZW, Meagher RJ, Williams KP (7 June 2014). "Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain". PLOS ONE. 9 (6): e99209. Bibcode:2014PLoSO...999209H. doi:10.1371/journal.pone.0099209. PMC 4048246. PMID 24905728.
- Lou Z, Sun Y, Rao Z (February 2014). "Current progress in antiviral strategies". Trends in Pharmacological Sciences. 35 (2): 86–102. doi:10.1016/j.tips.2013.11.006. PMC 7112804. PMID 24439476.
- Pennings PS (June 2013). "HIV Drug Resistance: Problems and Perspectives". Infectious Disease Reports. 5 (Suppl 1): e5. doi:10.4081/idr.2013.s1.e5. PMC 3892620. PMID 24470969.
- Das K, Arnold E (April 2013). "HIV-1 reverse transcriptase and antiviral drug resistance. Part 1". Current Opinion in Virology. 3 (2): 111–8. doi:10.1016/j.coviro.2013.03.012. PMC 4097814. PMID 23602471.
- Ton Q, Frenkel L (March 2013). "HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission". Current HIV Research. 11 (2): 126–36. doi:10.2174/1570162x11311020005. PMID 23432488.
- Ebrahim O, Mazanderani AH (June 2013). "Recent developments in hiv treatment and their dissemination in poor countries". Infectious Disease Reports. 5 (Suppl 1): e2. doi:10.4081/idr.2013.s1.e2. PMC 3892621. PMID 24470966.
- Xie JL, Polvi EJ, Shekhar-Guturja T, Cowen LE (2014). "Elucidating drug resistance in human fungal pathogens". Future Microbiology. 9 (4): 523–42. doi:10.2217/fmb.14.18. PMID 24810351.
- Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK (March 2014). "Overcoming antifungal resistance". Drug Discovery Today. Technologies. 11: 65–71. doi:10.1016/j.ddtec.2014.02.005. PMC 4031462. PMID 24847655.
- Costa C, Dias PJ, Sá-Correia I, Teixeira MC (2014). "MFS multidrug transporters in pathogenic fungi: do they have real clinical impact?". Frontiers in Physiology. 5: 197. doi:10.3389/fphys.2014.00197. PMC 4035561. PMID 24904431.
- Andrews KT, Fisher G, Skinner-Adams TS (August 2014). "Drug repurposing and human parasitic protozoan diseases". International Journal for Parasitology. Drugs and Drug Resistance. 4 (2): 95–111. doi:10.1016/j.ijpddr.2014.02.002. PMC 4095053. PMID 25057459.
- Visser BJ, van Vugt M, Grobusch MP (October 2014). "Malaria: an update on current chemotherapy". Expert Opinion on Pharmacotherapy. 15 (15): 2219–54. doi:10.1517/14656566.2014.944499. PMID 25110058. S2CID 34991324.
- Chia WN, Goh YS, Rénia L (2014). "Novel approaches to identify protective malaria vaccine candidates". Frontiers in Microbiology. 5: 586. doi:10.3389/fmicb.2014.00586. PMC 4233905. PMID 25452745.
- Franco JR, Simarro PP, Diarra A, Jannin JG (2014). "Epidemiology of human African trypanosomiasis". Clinical Epidemiology. 6: 257–75. doi:10.2147/CLEP.S39728. PMC 4130665. PMID 25125985.
- Herrera L (2014). "Trypanosoma cruzi, the Causal Agent of Chagas Disease: Boundaries between Wild and Domestic Cycles in Venezuela". Frontiers in Public Health. 2: 259. doi:10.3389/fpubh.2014.00259. PMC 4246568. PMID 25506587.
- Mansueto P, Seidita A, Vitale G, Cascio A (2014). "Leishmaniasis in travelers: a literature review" (PDF). Travel Medicine and Infectious Disease. 12 (6 Pt A): 563–81. doi:10.1016/j.tmaid.2014.09.007. hdl:10447/101959. PMID 25287721.
- Aminov RI (2010). "A brief history of the antibiotic era: lessons learned and challenges for the future". Frontiers in Microbiology. 1: 134. doi:10.3389/fmicb.2010.00134. PMC 3109405. PMID 21687759.
- Carvalho G, Forestier C, Mathias JD (December 2019). "Antibiotic resilience: a necessary concept to complement antibiotic resistance?". Proceedings. Biological Sciences. 286 (1916): 20192408. doi:10.1098/rspb.2019.2408. PMC 6939251. PMID 31795866.
- Organization, World Health (2014). Antimicrobial resistance : global report on surveillance. World Health Organization. Geneva, Switzerland. ISBN 9789241564748. OCLC 880847527.
- Amábile-Cuevas CF, editor. Antimicrobial resistance in bacteria. Horizon Scientific Press; 2007
- Fleming A (11 December 1945), "Penicillin" (PDF), Nobel Lecture, archived (PDF) from the original on 31 March 2018, retrieved 9 August 2020
- "WHO | Global action plan on antimicrobial resistance". WHO. Archived from the original on 18 April 2018. Retrieved 23 April 2018.
- Pollack A (20 January 2016). "To Fight 'Superbugs,' Drug Makers Call for Incentives to Develop Antibiotics". New York Times. Davos 2016 Special Report. Davos, Switzerland. Archived from the original on 24 April 2018. Retrieved 24 January 2016.
- Behdinan A, Hoffman SJ, Pearcey M (2015). "Some Global Policies for Antibiotic Resistance Depend on Legally Binding and Enforceable Commitments". The Journal of Law, Medicine & Ethics. 43 Suppl 3 (2): 68–73. doi:10.1111/jlme.12277. PMID 26243246. S2CID 7415203.
- Hoffman SJ, Outterson K (2015). "What Will It Take to Address the Global Threat of Antibiotic Resistance?". The Journal of Law, Medicine & Ethics. 43 (2): 363–8. doi:10.1111/jlme.12253. PMID 26242959. S2CID 41987305.
- Rizvi Z, Hoffman SJ (2015). "Effective Global Action on Antibiotic Resistance Requires Careful Consideration of Convening Forums". The Journal of Law, Medicine & Ethics. 43 Suppl 3 (2): 74–8. doi:10.1111/jlme.12278. PMID 26243247. S2CID 24223063.
- Andresen S, Hoffman SJ (2015). "Much Can Be Learned about Addressing Antibiotic Resistance from Multilateral Environmental Agreements". Journal of Law, Medicine & Ethics. 43 (2): 46–52.
- President’s 2016 Budget Proposes Historic Investment to Combat Antibiotic-Resistant Bacteria to Protect Public Health Archived 11 March 2015 at the Wayback Machine The White House, Office of the Press Secretary, 27 January 2015
- "FACT SHEET: Obama Administration Releases National Action Plan to Combat Antibiotic-Resistant Bacteria". whitehouse.gov. 27 March 2015. Archived from the original on 22 November 2015. Retrieved 30 October 2015.
- "Patients contracted 165 antibiotic resistant infections each day in 2018, says PHE". Pharmaceutical Journal. 31 October 2019. Retrieved 11 December 2019.
- Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW (September 2014). "Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department". The Cochrane Database of Systematic Reviews. 9 (9): CD006452. doi:10.1002/14651858.CD006452.pub4. PMC 6718218. PMID 25222468.
- Mishra RP, Oviedo-Orta E, Prachi P, Rappuoli R, Bagnoli F (October 2012). "Vaccines and antibiotic resistance". Current Opinion in Microbiology. 15 (5): 596–602. doi:10.1016/j.mib.2012.08.002. PMID 22981392.
- "Immunity, Infectious Diseases, and Pandemics—What You Can Do". HomesteadSchools.com. Archived from the original on 3 December 2013. Retrieved 12 June 2013.
- Shinefield, Henry; Black, Steven; Fattom, Ali; Horwith, Gary; Rasgon, Scott; Ordonez, Juan; Yeoh, Hock; Law, David; Robbins, John B.; Schneerson, Rachel; Muenz, Larry (14 February 2002). "Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis". The New England Journal of Medicine. 346 (7): 491–496. doi:10.1056/NEJMoa011297. ISSN 1533-4406. PMID 11844850.
- Fowler, Vance G.; Proctor, Richard A. (May 2014). "Where Does a Staphylococcus aureus Vaccine Stand?". Clinical Microbiology and Infection. 20 (5): 66–75. doi:10.1111/1469-0691.12570. ISSN 1198-743X. PMC 4067250. PMID 24476315.
- McNeely, Tessie B.; Shah, Najaf A.; Fridman, Arthur; Joshi, Amita; Hartzel, Jonathan S.; Keshari, Ravi S.; Lupu, Florea; DiNubile, Mark J. (2014). "Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors". Human Vaccines & Immunotherapeutics. 10 (12): 3513–3516. doi:10.4161/hv.34407. ISSN 2164-554X. PMC 4514053. PMID 25483690.
- Kim S, Lieberman TD, Kishony R (October 2014). "Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance". Proceedings of the National Academy of Sciences of the United States of America. 111 (40): 14494–9. Bibcode:2014PNAS..11114494K. doi:10.1073/pnas.1409800111. PMC 4210010. PMID 25246554.
- Pál C, Papp B, Lázár V (July 2015). "Collateral sensitivity of antibiotic-resistant microbes". Trends in Microbiology. 23 (7): 401–7. doi:10.1016/j.tim.2015.02.009. PMC 5958998. PMID 25818802.
- Imamovic L, Sommer MO (September 2013). "Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development". Science Translational Medicine. 5 (204): 204ra132. doi:10.1126/scitranslmed.3006609. PMID 24068739.
- Imamovic L, Ellabaan MM, Dantas Machado AM, Citterio L, Wulff T, Molin S, et al. (January 2018). "Drug-Driven Phenotypic Convergence Supports Rational Treatment Strategies of Chronic Infections". Cell. 172 (1–2): 121–134.e14. doi:10.1016/j.cell.2017.12.012. PMC 5766827. PMID 29307490.
- Liu J, Bedell TA, West JG, Sorensen EJ (June 2016). "Design and Synthesis of Molecular Scaffolds with Anti-infective Activity". Tetrahedron. 72 (25): 3579–3592. doi:10.1016/j.tet.2016.01.044. PMC 4894353. PMID 27284210.
- "Annual Report of the Chief Medical Officer - Infections and the rise of antimicrobial resistance" (PDF). UK NHS. 2011. Archived from the original (PDF) on 30 October 2013.
- "Obama Administration Seeks To Ease Approvals For Antibiotics". NPR. 4 June 2013. Archived from the original on 13 March 2015. Retrieved 7 August 2016.
- "Moldova Grapples With Whether To Isolate TB Patients". NPR. 4 June 2013. Archived from the original on 3 August 2016. Retrieved 7 August 2016.
- Walsh F (11 March 2013). "BBC News – Antibiotics resistance 'as big a risk as terrorism' – medical chief". BBC News. Bbc.co.uk. Archived from the original on 8 August 2018. Retrieved 12 March 2013.
- Khor M (18 May 2014). "Why Are Antibiotics Becoming Useless All Over the World?". The Real News. Archived from the original on 18 May 2014. Retrieved 18 May 2014.
- Nordrum A (2015). "Antibiotic Resistance: Why Aren't Drug Companies Developing New Medicines to Stop Superbugs?". International Business Times.
- Gever J (4 February 2011). "Pfizer Moves May Dim Prospect for New Antibiotics". MedPage Today. Archived from the original on 14 December 2013. Retrieved 12 March 2013.
- Ledford H (December 2012). "FDA under pressure to relax drug rules". Nature. 492 (7427): 19. Bibcode:2012Natur.492...19L. doi:10.1038/492019a. PMID 23222585.
- Office of the Press Secretary (18 September 2014). "Executive Order – Combating Antibiotics-Resistant Bacteria". The White House. Archived from the original on 22 September 2014. Retrieved 22 September 2014.
- President's Council of Advisors on Science and Technology (September 2014). "Report to the President on Combating Antibiotic Resistance" (PDF). PCAST. Archived (PDF) from the original on 22 September 2014. Retrieved 22 September 2014.
- Ventola, C. Lee (April 2015). "The antibiotic resistance crisis: part 1: causes and threats". P & T: A Peer-Reviewed Journal for Formulary Management. 40 (4): 277–283. ISSN 1052-1372. PMC 4378521. PMID 25859123.
- Leppäranta, Outi; Vaahtio, Minna; Peltola, Timo; Zhang, Di; Hupa, Leena; Hupa, Mikko; Ylänen, Heimo; Salonen, Jukka I.; Viljanen, Matti K.; Eerola, Erkki (1 February 2008). "Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro". Journal of Materials Science: Materials in Medicine. 19 (2): 547–551. doi:10.1007/s10856-007-3018-5. ISSN 1573-4838. PMID 17619981. S2CID 21444777.
- Munukka, Eveliina; Leppäranta, Outi; Korkeamäki, Mika; Vaahtio, Minna; Peltola, Timo; Zhang, Di; Hupa, Leena; Ylänen, Heimo; Salonen, Jukka I.; Viljanen, Matti K.; Eerola, Erkki (January 2008). "Bactericidal effects of bioactive glasses on clinically important aerobic bacteria". Journal of Materials Science: Materials in Medicine. 19 (1): 27–32. doi:10.1007/s10856-007-3143-1. ISSN 0957-4530. PMID 17569007. S2CID 39643380.
- Drago, Lorenzo; Vecchi, Elena De; Bortolin, Monica; Toscano, Marco; Mattina, Roberto; Romanò, Carlo Luca (August 2015). "Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains". Future Microbiology. 10 (8): 1293–1299. doi:10.2217/FMB.15.57. ISSN 1746-0913. PMID 26228640.
- Connelly, Erin (18 April 2017). "Medieval medical books could hold the recipe for new antibiotics". The Conversation.
- "AncientBiotics – a medieval remedy for modern day superbugs?" (Press release). University of Nottingham. 30 March 2015.
- Hopkins H, Bruxvoort KJ, Cairns ME, Chandler CI, Leurent B, Ansah EK, et al. (March 2017). "Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomised studies in public and private healthcare settings". BMJ. 356: j1054. doi:10.1136/bmj.j1054. PMC 5370398. PMID 28356302.
- "Diagnostics Are Helping Counter Antimicrobial Resistance, But More Work Is Needed". MDDI Online. 20 November 2018. Retrieved 2 December 2018.
- van Belkum A, Bachmann TT, Lüdke G, Lisby JG, Kahlmeter G, Mohess A, et al. (January 2019). "Developmental roadmap for antimicrobial susceptibility testing systems". Nature Reviews. Microbiology. 17 (1): 51–62. doi:10.1038/s41579-018-0098-9. hdl:2445/132505. PMC 7138758. PMID 30333569.
- "Progress on antibiotic resistance". Nature. 562 (7727): 307. October 2018. Bibcode:2018Natur.562Q.307.. doi:10.1038/d41586-018-07031-7. PMID 30333595.
- "Silent Killers: Fantastic Phages?". Archived from the original on 10 February 2013. Retrieved 14 November 2017.
- McAuliffe, et al. (2007). "The New Phage Biology: From Genomics to Applications" (introduction)". In McGrath S, van Sinderen D (eds.). Bacteriophage: Genetics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-14-1.
- Lin DM, Koskella B, Lin HC (August 2017). "Phage therapy: An alternative to antibiotics in the age of multi-drug resistance". World Journal of Gastrointestinal Pharmacology and Therapeutics. 8 (3): 162–173. doi:10.4292/wjgpt.v8.i3.162. PMC 5547374. PMID 28828194.
- Salmond GP, Fineran PC (December 2015). "A century of the phage: past, present and future". Nature Reviews. Microbiology. 13 (12): 777–86. doi:10.1038/nrmicro3564. PMID 26548913. S2CID 8635034.
- Letarov AV, Golomidova AK, Tarasyan KK (April 2010). "Ecological basis for rational phage therapy". Acta Naturae. 2 (1): 60–72. doi:10.32607/20758251-2010-2-1-60-71. PMC 3347537. PMID 22649629.
- Parfitt T (June 2005). "Georgia: an unlikely stronghold for bacteriophage therapy". Lancet. 365 (9478): 2166–7. doi:10.1016/S0140-6736(05)66759-1. PMID 15986542. S2CID 28089251.
- Golkar Z, Bagasra O, Pace DG (February 2014). "Bacteriophage therapy: a potential solution for the antibiotic resistance crisis". Journal of Infection in Developing Countries. 8 (2): 129–36. doi:10.3855/jidc.3573. PMID 24518621.
- McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, et al. (September 2013). "Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects". Virology. 443 (2): 187–96. doi:10.1016/j.virol.2013.05.022. PMID 23755967.
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (March 2011). "Phage treatment of human infections". Bacteriophage. 1 (2): 66–85. doi:10.4161/bact.1.2.15845. PMC 3278644. PMID 22334863.
- Onsea, Jolien; Soentjens, Patrick; Djebara, Sarah; Merabishvili, Maia; Depypere, Melissa; Spriet, Isabel; De Munter, Paul; Debaveye, Yves; Nijs, Stefaan; Vanderschot, Paul; Wagemans, Jeroen (23 September 2019). "Bacteriophage Application for Difficult-To-Treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol". Viruses. 11 (10): 891. doi:10.3390/v11100891. ISSN 1999-4915. PMC 6832313. PMID 31548497.
Books
- Caldwell R, Lindberg D, eds. (2011). "Understanding Evolution" [Mutations are random]. University of California Museum of Paleontology.
- Reynolds LA; Tansey EM, eds. (2008). Superbugs and superdrugs : a history of MRSA : the transcript of a Witness Seminar held by the Wellcome Trust Centre for the History of Medicine at UCL, London, on 11 July 2006. London: Wellcome Trust Centre for the History of Medicine at UCL. ISBN 978-0-85484-114-1.
- OECD (2018), Stemming the Superbug Tide: Just A Few Dollars More, OECD Health Policy Studies, OECD Publishing, Paris, https://doi.org/10.1787/9789264307599-en.
Journals
- Anomaly J (2017). "Antibiotics and Public Policy" (PDF). Georgetown University Press.
- Arias CA, Murray BE (January 2009). "Antibiotic-resistant bugs in the 21st century--a clinical super-challenge". The New England Journal of Medicine. 360 (5): 439–43. doi:10.1056/NEJMp0804651. PMID 19179312. S2CID 205104375.
- "Special Issue: Ethics and Antimicrobial Resistance". Bioethics. 365 (33). 2019.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M (2005). Esac Project. "Outpatient antibiotic use in Europe and association with resistance: a cross-national database study". Lancet. Group Esac Project. 365 (9459): 579–87. doi:10.1016/S0140-6736(05)17907-0. PMID 15708101. S2CID 23782228.
- Hawkey PM, Jones AM (September 2009). "The changing epidemiology of resistance" (PDF). The Journal of Antimicrobial Chemotherapy. 64 Suppl 1: i3-10. doi:10.1093/jac/dkp256. PMID 19675017.
- Soulsby EJ (November 2005). "Resistance to antimicrobials in humans and animals". BMJ. 331 (7527): 1219–20. doi:10.1136/bmj.331.7527.1219. PMC 1289307. PMID 16308360.
- "Alternatives to Antibiotics Reduce Animal Disease". Commonwealth Scientific and Industrial Research Organization. 9 January 2006.
- Cooke P, Rees-Roberts D (2017). CATCH. 16-minute film about a post-antibiotic world. Review: Sansom C (March 2017). "Media Watch: An intimate family story in a world without antibiotics". Lancet Infect Dis. 17 (3): 274. doi:10.1016/S1473-3099(17)30067-1.
External links
| Wikimedia Commons has media related to Antibiotic resistance. |
- Antimicrobial resistance at Curlie
- Animation of Antibiotic Resistance
- CDC Guideline "Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006"
- Antimicrobial Stewardship Project, at the Center for Infectious Disease Research and Policy (CIDRAP), University of Minnesota
- AMR Industry Alliance, "members from large R&D pharma, generic manufacturers, biotech, and diagnostic companies"