Asymmetric dimethylarginine
Asymmetric dimethylarginine (ADMA) is a naturally occurring chemical found in blood plasma. It is a metabolic by-product of continual protein modification processes in the cytoplasm of all human cells. It is closely related to L-arginine, a conditionally essential amino acid. ADMA interferes with L-arginine in the production of nitric oxide (NO), a key chemical involved in normal endothelial function and, by extension, cardiovascular health.
 | |
 | |
| Names | |
|---|---|
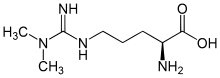
| IUPAC name
2-Amino-5-[[amino(dimethylamino)methylidene]amino]pentanoic acid | |
| Other names
N(G),N(G′)-Dimethylarginine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 2261521 S | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | N,N-dimethylarginine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H18N4O2 | |
| Molar mass | 202.258 g·mol−1 |
| log P | −0.716 |
| Acidity (pKa) | 2.497 |
| Basicity (pKb) | 11.500 |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery
Patrick Vallance and his London co-workers first noted the interference role for asymmetric dimethylarginine in the early 1990s.[1] Today biochemical and clinical research continues into the role of ADMA in cardiovascular disease, diabetes mellitus, erectile dysfunction and certain forms of kidney disease.
Synthesis and regulation in the body
Asymmetric dimethylarginine is created in protein methylation, a common mechanism of post-translational protein modification. This reaction is catalyzed by an enzyme set called S-adenosylmethionine protein N-methyltransferases (protein methylases I and II).[2] The methyl groups transferred to create ADMA are derived from the methyl group donor S-adenosylmethionine, an intermediate in the metabolism of homocysteine. (Homocysteine is an important blood chemical because it is also a marker of cardiovascular disease). After synthesis, ADMA migrates into the extracellular space and thence into blood plasma. Asymmetric dimethylarginine is measured using high-performance liquid chromatography.
ADMA concentrations are substantially elevated by native or oxidized LDL cholesterol.[3] Thus a spiralling effect occurs with high endothelial LDL levels causing greater ADMA values, which in turn inhibit NO production needed to promote vasodilation. The elimination of ADMA occurs through urine excretion and metabolism by the enzyme dimethylarginine dimethylaminohydrolase (DDAH). The role of homocysteine as a risk factor for cardiovascular disease is suggested to be mediated by homocysteine down-regulating production of DDAH in the body. Polyphenol antioxidants also play a role in down-regulating homocysteine.
ADMA and suggested lines of therapeutic research
With raised levels of ADMA seemingly to be associated with adverse human health consequences for cardiovascular disease, metabolic diseases, and also a wide range of diseases of the elderly, the possible lowering of ADMA levels may have important therapeutic effects. However, it has yet to be established whether ADMA levels can be manipulated and, more important, if this results in useful clinical benefits.
The association of ADMA with abnormalities of lipid regulation suggested that supplements of free fatty acids might manipulate ADMA levels. However, research has failed to show that these have an effect.[4][5]
ADMA's role has been linked with elevated levels of homocysteine.[6][7][8] Whilst approaches at modifying the latter with oral supplements of folic acid were strongly suggested, studies have shown this fails to give any clinical benefit and suggested that B vitamins might instead increase some cardiovascular risks.[9][10][11]
Direct alteration of ADMA levels with supplements of L-arginine have been suggested.[12][13] The hope is that such intervention might not only improve endothelial function but also reduce clinical symptoms of overt cardiovascular disease.[14][15] However studies show inconsistency in results in a clinical context,[16] and the recent results with manipulating homocysteine levels warrant extreme care with what clinical outcomes might arise from this approach.
Statins, as well as affecting circulating cholesterol levels, also increase nitric oxide levels and so have a direct effect on blood supply to the heart. Elevated levels of ADMA seems to modify this effect and so may have consequences for patients' responsiveness to taking statins.[17]
Repeated administration of d-amphetamine may decrease ADMA.[18]
References
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. (1992). "Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis". Journal of Cardiovascular Pharmacology. 20 Suppl 12: S60–S62. doi:10.1097/00005344-199204002-00018. PMID 1282988. S2CID 38113918.
- Rawal N, Rajpurohit R, Lischwe MA, Williams KR, Paik WK, Kim S (1995). "Structural specificity of substrate for S-adenosylmethionine:protein arginine N-methyltransferases". Biochim Biophys Acta. 1248 (1): 11–8. doi:10.1016/0167-4838(94)00213-Z. PMID 7536038.
- Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM (21 July 2000). "LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases". Circ Res. 87 (2): 99–105. doi:10.1161/01.RES.87.2.99. PMID 10903992.
- Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Ellingsen I, Seljeflot I (2006). "Effect of diet and omega-3 fatty acid intervention on asymmetric dimethylarginine". Nutr Metab (Lond). 3 (1): 4. doi:10.1186/1743-7075-3-4. PMC 1343562. PMID 16396682.
- Namiranian K, Mittermayer F, Artwohl M, Pleiner J, Schaller G, Mayer BX, Bayerle-Eder M, Roden M, Baumgartner-Parzer S, Wolzt M (2005). "Free fatty acids do not acutely increase asymmetrical dimethylarginine concentrations". Horm Metab Res. 37 (12): 768–72. doi:10.1055/s-2005-921100. PMID 16372232.
- Krzyzanowska K, Mittermayer F, Krugluger W, Schnack C, Hofer M, Wolzt M, Schernthaner G (2006). "Asymmetric dimethylarginine is associated with macrovascular disease and total homocysteine in patients with type 2 diabetes". Atherosclerosis. 189 (1): 236–40. doi:10.1016/j.atherosclerosis.2005.12.007. PMID 16414052.
- Dayal S, Lentz SR (2005). "ADMA and hyperhomocysteinemia". Vasc Med. 10 Suppl 1 (2): S27–33. doi:10.1191/1358863x05vm599oa. PMID 16444866.
- Stuhlinger MC, Stanger O (2005). "Asymmetric dimethyl-L-arginine (ADMA): a possible link between homocyst(e)ine and endothelial dysfunction". Curr Drug Metab. 6 (1): 3–14. doi:10.2174/1389200052997393. PMID 15720202.
- Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ (2006). "Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial". J Am Coll Cardiol. 47 (6): 1108–16. doi:10.1016/j.jacc.2005.10.064. PMID 16545638.
- Lonn, E; Yusuf, S; Arnold, MJ; Sheridan, P; Pogue, J; Micks, M; McQueen, MJ; Probstfield, J; et al. (2006). "Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease". N Engl J Med. 354 (15): 1567–77. doi:10.1056/NEJMoa060900. PMID 16531613. S2CID 21790155.
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K (2006). "Homocysteine Lowering and Cardiovascular Events after Acute Myocardial Infarction". N Engl J Med. 354 (15): 1578–88. doi:10.1056/NEJMoa055227. PMID 16531614.
- Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC (2003). "Oral L-arginine improves endothelial function in healthy individuals older than 70 years". Vasc Med. 8 (2): 77–81. doi:10.1191/1358863x03vm474oa. PMID 14518608.
- John P. Cooke (2002). The Cardiovascular Cure. Random House. ISBN 0-7679-0881-3.
- Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH (15 June 1996). "Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure". Circulation. 93 (12): 2135–41. doi:10.1161/01.CIR.93.12.2135. PMID 8925582.
- Ceremuzynski L, Chamiec T, Herbaczynska-Cedro K (1997). "Effect of supplemental oral L-arginine on exercise capacity in patients with stable angina pectoris". Am J Cardiol. 80 (3): 331–3. doi:10.1016/S0002-9149(97)00354-8. PMID 9264427.
- Loscalzo J (2004). "L-arginine and atherothrombosis". J Nutr. 134 (10 Suppl): 2798S–2800S, discussion 2818S–2819S. doi:10.1093/jn/134.10.2798S. PMID 15465788.
- Janatuinen T, Laakso J, Laaksonen R, Vesalainen R, Nuutila P, Lehtimaki T, Raitakari OT, Knuuti J (2003). "Plasma asymmetric dimethylarginine modifies the effect of pravastatin on myocardial blood flow in young adults". Vasc Med. 8 (3): 185–9. doi:10.1191/1358863x03vm490oa. PMID 14989559. S2CID 36938530.
- Vanaveski, Taavi; Narvik, Jane; Innos, Jürgen; Philips, Mari-Anne; Ottas, Aigar; Plaas, Mario; Haring, Liina; Zilmer, Mihkel; Vasar, Eero (2018-06-12). "Repeated Administration of D-Amphetamine Induces Distinct Alterations in Behavior and Metabolite Levels in 129Sv and Bl6 Mouse Strains". Frontiers in Neuroscience. 12: 399. doi:10.3389/fnins.2018.00399. ISSN 1662-4548. PMC 6005828. PMID 29946233.