Bactrocera dorsalis
Bactrocera dorsalis, previously known as Dacus dorsalis and commonly referred to as the oriental fruit fly, is a species of tephritid fruit fly that is endemic to Southeast Asia. It is one of the major pest species in the genus Bactrocera with a broad host range of cultivated and wild fruits.[1] Male B. dorsalis respond strongly to methyl eugenol, which is used to monitor and estimate populations, as well as to annihilate males as a form of pest control.[2][3][4] They are also important pollinators and visitors of wild orchids, Bulbophyllum cheiri and Bulbophyllum vinaceum in Southeast Asia, which lure the flies using methyl eugenol.[5][6]
| Bactrocera dorsalis | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Subgenus: | Bactrocera |
| Species: | B. dorsalis |
| Binomial name | |
| Bactrocera dorsalis (Hendel, 1912) | |
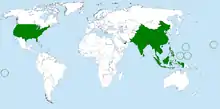 | |
| Bactrocera dorsalis range (by country) | |
| Synonyms | |
|
Dacus dorsalis | |
The fly is similar to the closely related species B. carambolae and B. occipitalis. The species name B. dorsalis is identical to other synonyms B. papayae, B. invadens and B. philippinensis.[7][6]
Description
_(06410590)_(6922916899).jpg.webp)
B. dorsalis is a species of tephritid fruit fly. Flies that belong to this family are usually small to medium-sized with colorful markings. In particular, B. dorsalis belongs to a complex of physically similar flies called the Bactrocera dorsalis complex, whose defining characteristics include a mostly black thorax and dark T-shaped marking on the fly's abdominal segment. The T-shape marking consists of a dark medial and transverse band along the fly's abdomen.[8]
The B. dorsalis species has distinctive yellow and black markings on its thorax and abdomen, which may vary between flies. Two vertical yellow markings on the thorax and the dark T-shaped marking on the abdomen differentiate this species of fly from its close relatives. The wings are clear with a continuous costal band. The adult body is around 8.0 mm in length, with wings approximately 7.3 mm in length. The female adult has a tapered ovipositor for depositing eggs in host fruits, while in male adults this ovipositor is notably absent.[9]
Distribution
Endemic to Southeast Asia, B. dorsalis is a highly invasive pest species that now has a presence in at least 65 countries. It has also been introduced to Hawaii, the Mariana Islands, and Tahiti. The fly is also found in most countries of sub-Saharan Africa.[9] From 1910 to 1990, the fly species was only observed in 5 countries; however, in the last three decades, the rate of spread by B. dorsalis has sharply increased, with the species invading an additional 70 countries.[10]
Elsewhere in the United States, B. dorsalis has been spotted in California and Florida. These appearances then trigger a cascade of eradication efforts. Four major oriental fruit fly eradication efforts occurred in response to infestations in California between 1960 and 1997. Two additional infestations were eradicated in 2006 and 2007, occurring within 3–4 years of reports of these infestations.[9] In July 2010, flies were discovered in traps in the Sacramento and Placer counties of California. A quarantine was established, and eradication efforts followed. These sightings in the mainland United States are generally quarantined infestations that have been eradicated.[11]
CLIMEX, a modeling software, has been used to map the future trajectory for the fruit fly in terms of opportunities for increasing its distribution.[12] This was tested both under current and future predicted climate conditions, given the current research on climate change. Under current conditions, the fly's projected distribution includes much of the tropics and subtropics and extends into areas like Mediterranean Europe. The model predicts optimal climate conditions in the southeast United States.[12] Under climate change conditions, the spread overall increases as the fly is less limited by cold weather. However, its distribution does possibly decrease in areas where precipitation decreases.[12]
Habitat
These tephritid fruit flies are found in tropical areas. B. dorsalis also prefers to pupate in shaded rather than brightly lit areas, moist over dry soil, and in soil with larger particles (particle size greater than or equal to 2.5 mm) than in soil with smaller particles.[13]
Life history
.jpg.webp)
Under summer conditions, development from egg to adult requires 16 days. Several stages can be delayed in cooler conditions. B. dorsalis eggs may take up to 20 days to hatch under cool conditions, extended from the usual single day.[11] Pupariation occurs in the soil under the host plant and is normally completed within 10–12 days, but can be delayed up to 90 days under cool conditions.[11] Flies typically live from 1–3 months, but this can be extended to up to 12 months under cool conditions.[11]
Eggs
Mated female B. dorsalis puncture the skin of mature fruit and deposit eggs in a few batches of 3-30 eggs underneath a fruit's skin via ovipositor, depending on the quality and ripeness of the fruit. Eggs usually hatch within a day, although the hatching process can be delayed in cool conditions. Females are capable of laying over 3,000 eggs during their lifetime under optimal conditions, but in field conditions, reports a typical range from about 1,200 to 1,500 eggs laid per female.[9]
Larvae
Eggs hatch to larvae and moult twice (there are three larval instars) while feeding on the flesh of the fruit for about 6–35 days. Larvae are creamy white in color with a maggot-like appearance and are about 10 mm in length.[14]
Pupae
The third instar larvae exit the fruit and burrow into the soil under the host plant to pupate. Larvae generally pupate in the upper 4 cm of the soil.[13] Pupation can take approximately 10–12 days, but depending on the season, this process can be delayed in cooler conditions.[14]
_(06410589)_(6776801024).jpg.webp)
Adults
In 1-2 weeks, the adult emerges from the pupae and matures. Most adults emerge from the soil between 7:00 AM and 10:00 AM. Once sexual maturity is reached (which takes approximately 9 days), adults engage in the mating process and the life cycle repeats. The adult lifespan for B. dorsalis is about 90 days, and the flies have been reported to travel up to 30 miles in search of new egg laying sites and food, such as decaying fruit and plant nectar. Adult females prefer to lay eggs in old egg deposit sites on fruits.[9][11][14][15]
Food resources
B. dorsalis has been seen in more than 200 kinds of fruit and nut plants, but the species lay eggs in mango, papaya, and avocado fruits most often. Adult flies feed on decaying fruit, plant nectar, and other substances during their lifetime and prefer to feed in the morning.[14][16]
Social behavior
Mating
Polyandry has been observed in B. dorsalis.[17] For females, there is typically a re-mating refractory period. The length of this period does not vary based on whether the female is mating with a virgin or non-virgin male. However, when there was a refractory period, females lay more eggs.[17] Females who were exposed to two males continuously without a refractory period in between lay fewer eggs, but still lay more eggs than females with only one male. Therefore, there appears to be a reproductive benefit for females with polyandry.[17]
Reproductive senescence does appear to be present in this species, as male and female age correlates negatively with the rate of fertilization.[17]
Flying
The flight capacity of B. dorsalis adult females at various life stages has been observed in order to better understand and prevent their spread. Past research has shown that the species can spread extensively following fruit harvests, with a dispersal radius up to 37 km observed in Hawaii.[18] Transmission electron microscopy was employed to view changes in flight muscle ultrastructures. Researchers observed that flight speed and distance changed with the age of the fruit fly, reaching its maximum capacity at 15 days of age.[18]
Long-distance flight
B. dorsalis has been observed to be capable of long-distance flight. One study investigated the relationship between flight muscle structure and the flies' flight capacity: as the number of mitochondria increased, myofibril diameter increased, and sarcomere length decreased, the researchers found the fly's flight capacity to be maximized. This particular muscle structure was evident in 15-day-old female adult B. dorsalis flies.[18]
Symbiotic relationships
B. dorsalis has symbiotic relationships with many bacteria. Different bacteria dominate at different developmental stages of the fruit fly.[19] Proteobacteria are most often present in immature stages, whereas Firmicutes are most often present in the adult stages.[19] Overall, the most abundant families are Enterococcaceae and Comamondaceae. Meanwhile, Comamonas are extremely abundant in pupae, but disappear entirely by adulthood.[19]
Researchers have also tested the relationship between certain gut symbiotic bacteria and fly foraging behavior and nutrient ingestion. Suppression of the fly's microbiome resulted in changes in the foraging behavior in both male and female flies.[20] Aposymbiotic flies responded faster to diets in experimental conditions and fed more, for longer periods of time.[20]
Interactions with humans
Human activities are partially responsible for causing the spread of the species from one region to another. The primary risk comes from import of fruit that may contain larvae, either in passenger cargo, or through the smuggling of fruit in passenger baggage or mail.[11] In New Zealand, researchers recorded 7-33 interceptions of fruit flies per year in cargo. Researchers also recorded an additional 10-28 interceptions in passenger baggage.[11]
Agricultural pest
B. dorsalis is not only a highly invasive species, accidentally introduced to Hawaii from Taiwan during the 1940s in World War II, but also very destructive to crop yield for farmers of various fruits, vegetables, and nuts across the world. The larval stage of the life cycle is the most damaging to fruits because of larval feeding on the soft flesh of fruits. After ovipositing occurs by a female fly, the larvae develop under the skin of the fruit or soft tissues of the plant and begin to feed on the fruit or plant's flesh. Once feeding occurs, other microorganisms can invade the site of larval feeding and cause the fruit to decay faster. Although ripe fruits are believed to be preferred for ovipositing, unripe fruits have served as hosts for eggs as well.[16]
Conventional pest treatment
B. dorsalis has created many agricultural issues for humans, especially in the areas where it is endemic. In the Pacific Islands, the fly has restricted the development of a diverse tropical fruit and vegetable industry, necessitated that commercial fruits go through quarantine treatment before shipment, and provided an avenue for their introduction to countries not previously exposed to the fly species.[21] The Miami-Dade County in Florida had to perform the quarantine technique when the insect was detected in fruits there in August 2015; they were eliminated from the area in February 2016.[22]
To resolve these concerns, several techniques have been implemented, including sterile insect technique, protein bait sprays, and male annihilation.[21] Male annihilation technique is effective because methyl eugenol attracts male flies prior to the beginning of their sexual maturation, to an extent of 40 to 50 percent of the flies.[4]
One of the most experimentally effective control techniques has been the wrapping of fruit, often in a paper or polythene sleeve. This physical barrier prevents oviposition from occurring.[11] The caveat with this method is that it must be implemented far in advance of the fruit fly's presence. Alternatively, fruits can be harvested earlier in the season than the flies anticipate; this has proven effective with the mango fruit.[11]

Parasitoid wasps
In addition to these, Hawaii has developed methods to suppress Bactrocera species using parasitoid wasps, including Fopius arisanus. The parasitic wasp oviposits its own eggs into B. dorsalis eggs, the parasitoids are reared in the host, and the developed parasitoids emerge in the pupal stage. F. arisanus has been observed to be the most successful example of parasitoid control of B. dorsalis, and researchers are looking to introduce its model of suppression from Hawaii to other areas of the world that the fly affects.[21]
Field sanitation
One simple, but effective technique called field sanitation, through which all unmarketable fruits get removed from the fields and disposed as soon as they are observed, so that re-infestation does not occur.[16]
Insecticide resistance
Many organophosphorus insecticides target the enzyme acetylcholinesterase (AChE); mutations in the acetylcholinesterase gene of B. dorsalis have been found to be associated to resistance to such insecticides. Researchers have identified three point mutations in B. dorsalis' gene encoding AChE that generate nonsynonymous changes in the produced amino acid sequence. Two of the point mutations are identical in site to mutations identified in other Bactrocera species, but one of the mutations is specific to B. dorsalis. Widespread use of such insecticides could result in rapid resistance acquisition in populations of B. dorsalis.[23]
References
- Drew RA, Raghu S (December 2002). "The fruit fly fauna (Diptera: Tephritidae: Dacinae) of the rainforest habitat of the Western Ghats, India" (PDF). Raffles Bulletin of Zoology. 50 (2): 327–52.
- Tan KH, Serit M (April 1994). "Adult population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in relation to host phenology and weather in two villages of Penang Island, Malaysia". Environmental Entomology. 23 (2): 267–75. doi:10.1093/ee/23.2.267.
- Hee AK, Tan KH (December 2005). "Bioactive fractions containing methyl eugenol-derived sex pheromonal components in haemolymph of the male fruit fly Bactrocera dorsalis (Diptera: Tephritidae)". Bulletin of Entomological Research. 95 (6): 615–20. doi:10.1079/BER2005392. PMID 16336709.
- Wong, Tim T. Y.; McInnis, Don O.; Nishimoto, Jon I. (1989-04-01). "Relationship of sexual maturation rate to response of oriental fruit fly strains (Diptera: Tephritidae) to methyl eugenol". Journal of Chemical Ecology. 15 (4): 1399–1405. doi:10.1007/BF01014839. ISSN 1573-1561. PMID 24272021.
- Tan KH, Nishida R, Toong YC (2002). "Bulbophyllum cheiri's floral synomone lures fruit flies to perform pollination". J. Chem. Ecol. 28 (6): 1161–72. doi:10.1023/A:1016277500007. PMID 12184394.
- Tan KH, Tan LT, Nishida R (November 2006). "Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination". Journal of Chemical Ecology. 32 (11): 2429–41. doi:10.1007/s10886-006-9154-4. PMID 17082990.
- Schutze MK, Aketarawong N, Amornsak W, Armstrong KF, Augustinos AA, Barr N, et al. (April 2015). "Synonymization of key pest species within the B actrocera dorsalis species complex (D iptera: T ephritidae): taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data". Systematic Entomology. 40 (2): 456–71. doi:10.1111/syen.12113.
- Leblanc L, San Jose M, Barr N, Rubinoff D (2015-11-26). "A phylogenetic assessment of the polyphyletic nature and intraspecific color polymorphism in the Bactrocera dorsalis complex (Diptera, Tephritidae)". ZooKeys (540): 339–67. doi:10.3897/zookeys.540.9786. PMC 4714077. PMID 26798267.
- "oriental fruit fly - Bactrocera dorsalis". entnemdept.ufl.edu. Retrieved 2019-10-01.
- Zeng, Yiying; Reddy, Gadi V. P.; Li, Zhihong; Qin, Yujia; Wang, Yannan; Pan, Xubin; Jiang, Fan; Gao, Feng; Zhao, Zi-Hua (2019-04-01). "Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae)". Journal of Applied Entomology. 143 (3): 165–176. doi:10.1111/jen.12582. ISSN 1439-0418.
- "Bactrocera dorsalis (Oriental fruit fly)". Invasive Species Compendium. July 9, 2019.
- Stephens AE, Kriticos DJ, Leriche A (August 2007). "The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae)" (PDF). Bulletin of Entomological Research. 97 (4): 369–78. doi:10.1017/S0007485307005044. PMID 17645818.
- Alyokhin, Andrei V.; Mille, Christian; Messing, Russell H.; Duan, Jian J. (2001-01-01). "Selection of Pupation Habitats by Oriental Fruit Fly Larvae in the Laboratory". Journal of Insect Behavior. 14 (1): 57–67. doi:10.1023/A:1007849629409. ISSN 1572-8889.
- "Oriental Fruit Fly Bactrocera dorsalis". Texas Invasive Species Institute. Retrieved 2019-10-01.
- Vargas RI, Walsh WA, Kanehisa D, Jang EB, Armstrong JW (1997-03-01). "Demography of Four Hawaiian Fruit Flies (Diptera: Tephritidae) Reared at Five Constant Temperatures". Annals of the Entomological Society of America. 90 (2): 162–168. doi:10.1093/aesa/90.2.162. ISSN 0013-8746.
- "Bactrocera dorsalis". Knowledge Master. University of Hawii. Retrieved 2019-10-01.
- Wei D, Feng YC, Wei DD, Yuan GR, Dou W, Wang JJ (2015). "Female remating inhibition and fitness of Bactrocera dorsalis (Diptera: Tephritidae) associated with male accessory glands". Florida Entomologist. 98 (1): 52–58. doi:10.1653/024.098.0110.
- Chen M, Chen P, Ye H, Yuan R, Wang X, Xu J (2015-10-08). "Flight capacity of Bactrocera dorsalis (Diptera: Tephritidae) adult females based on flight mill studies and flight muscle ultrastructure". Journal of Insect Science. 15 (1): 141. doi:10.1093/jisesa/iev124. PMC 4626671. PMID 26450591.
- Andongma AA, Wan L, Dong YC, Li P, Desneux N, White JA, Niu CY (March 2015). "Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis". Scientific Reports. 5: 9470. Bibcode:2015NatSR...5E9470A. doi:10.1038/srep09470. PMC 5380164. PMID 25822599.
- Akami M, Andongma AA, Zhengzhong C, Nan J, Khaeso K, Jurkevitch E, et al. (2019-01-16). "Intestinal bacteria modulate the foraging behavior of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae)". PLOS ONE. 14 (1): e0210109. Bibcode:2019PLoSO..1410109A. doi:10.1371/journal.pone.0210109. PMC 6334898. PMID 30650116.
- Vargas RI, Leblanc L, Harris EJ, Manoukis NC (August 2012). "Regional Suppression of Bactrocera Fruit Flies (Diptera: Tephritidae) in the Pacific through Biological Control and Prospects for Future Introductions into Other Areas of the World". Insects. 3 (3): 727–42. doi:10.3390/insects3030727. PMC 4553587. PMID 26466626.
- "Florida Says Its Fruits, Vegetables Are Safe from Invasive Fruit Fly".
- Hsu, Ju-Chun; Haymer, David S.; Wu, Wen-Jer; Feng, Hai-Tung (2006-05-01). "Mutations in the acetylcholinesterase gene of Bactrocera dorsalis associated with resistance to organophosphorus insecticides". Insect Biochemistry and Molecular Biology. 36 (5): 396–402. doi:10.1016/j.ibmb.2006.02.002. ISSN 0965-1748. PMID 16651186.
Further reading
- Allwood, A.J., Chinajariyawong, A., Drew, R.A.I., et al. (1999) Host plant records for fruit flies (Diptera: Tephritidae) in south east Asia. Raffles Bulletin of Zoology Supplement 7:1-92.
External links
- oriental fruit fly on the UF / IFAS Featured Creatures Web site
- Species Profile- Oriental Fruit Fly (Bactrocera dorsalis), National Invasive Species Information Center, United States National Agricultural Library. Lists general information and resources for Oriental Fruit Fly.