Bixin
Bixin is an apocarotenoid found in annatto, a natural food coloring obtained from the seeds of the achiote tree (Bixa orellana). Annatto seeds contain about 5% pigments, which consist of 70-80% bixin.[2]
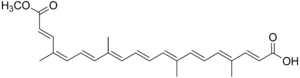 | |
.png.webp) | |
| Names | |
|---|---|
| IUPAC name
(2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acid | |
| Other names
cis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.499 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C25H30O4 | |
| Molar mass | 394.511 g·mol−1 |
| Appearance | Orange crystals |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bixin is chemically unstable when isolated and converts via isomerization into trans-bixin (β-bixin), the double-bond isomer.[1]
Bixin is soluble in fats and alcohols but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
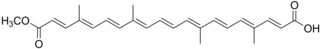 Chemical structure of trans-bixin
Chemical structure of trans-bixin.png.webp) Ball-and-stick model of trans-bixin
Ball-and-stick model of trans-bixin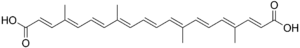 Chemical structure of norbixin
Chemical structure of norbixin red seeds of the achiote tree
red seeds of the achiote tree
References
- Merck Index, 11th Edition, 1320
- Executive Summary Bixin Archived July 21, 2011, at the Wayback Machine, National Toxicology Program
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
