Tazarotene
Tazarotene (marketed as Tazorac, Avage, Zorac, and Fabior) is a third-generation prescription topical retinoid sold as a cream, gel, or foam. Tazarotene is a member of the acetylenic class of retinoids. This medication is approved for treatment of psoriasis, acne, and sun damaged skin (photodamage). It is commonly sold in two concentrations: 0.05% and 0.1%.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tazorac |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >99% |
| Elimination half-life | 19 Hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.115.380 |
| Chemical and physical data | |
| Formula | C21H21NO2S |
| Molar mass | 351.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
Tazarotene is most commonly used topically to treat acne vulgaris and psoriasis. It can also be used to reduce skin discoloration and facial wrinkling.[1]
Tazarotene is rated pregnancy category X, and should not be used by pregnant women.
Available forms
Tazarotene is dispensed as a topical medication, either as a cream, gel, lotion, or foam.[2]
Side effects
Side effects for tazarotene include skin irritation, such as redness, itchiness, and burning. In patients with psoriasis, these side effects can be mitigated by a combined treatment with either mometasone furoate or fluocinonide.[3]
Synthesis
Acetylenic retinoid prodrug converted to the active metabolite, tazarotenic acid, with selective affinity for retinoic acid receptors RARβ and RARγ.
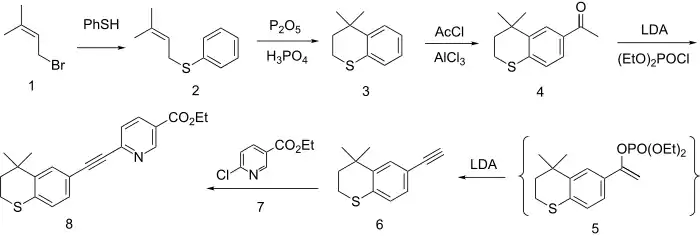
The formation of the ring system involves first alkylation of the anion from thiophenol with dimethylallyl bromide (1) to give the thioether (2). Friedel-Crafts cyclization of the olefin with the equivalent of PPA then gives the thiopyran (3). Acylation with acetyl chloride in the presence of aluminium chloride gives the methyl ketone (4). Reaction of the enolate of that ketone with diethyl chlorophosphate gives the enol phosphate 5 as a transient intermediate. This eliminates diethyl phosphite in the presence of excess base to give the corresponding acetylene 6. The anion from the reaction of the acetylene with base is then used to displace chlorine from Ethyl 6-chloronicotinate (7). This reaction affords the coupling product tazarotene (8).
Notes
- American Society of Health-System Pharmacists, Inc. "Tazarotene". MedlinePlus Drug Information. U.S. National Library of Medicine. Retrieved 2017-09-16.
- "What are the different brands of tazarotene?". Drugs.com. Retrieved November 6, 2020.
- Foster RH, Brogden RN, Benfield P (May 1998). "Tazarotene". Drugs. 55 (5): 705–11, discussion 712. doi:10.2165/00003495-199855050-00008. PMID 9585866.
- Prepn: Chandraratna RA, EP 284288; idem, U.S. Patent 5,089,509 (1988, 1992 both to Allergan).