Astaxanthin
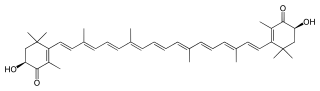
Astaxanthin /æstəˈzænθɪn/ is a keto-carotenoid.[3][4] It belongs to a larger class of chemical compounds known as terpenes (as a tetraterpenoid) built from five carbon precursors, isopentenyl diphosphate, and dimethylallyl diphosphate. Astaxanthin is classified as a xanthophyll (originally derived from a word meaning "yellow leaves" since yellow plant leaf pigments were the first recognized of the xanthophyll family of carotenoids), but currently employed to describe carotenoid compounds that have oxygen-containing components, hydroxyl (-OH) or ketone (C=O), such as zeaxanthin and canthaxanthin. Indeed, astaxanthin is a metabolite of zeaxanthin and/or canthaxanthin, containing both hydroxyl and ketone functional groups.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,3′-dihydroxy-β,β-carotene-4,4′-dione | |
| Systematic IUPAC name
(6S)-6-Hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone | |
| Other names
β-Carotene-4,4'-dione, 3,3'-dihydroxy-, all-trans-; (3S,3'S)-Astaxanthin; (3S,3'S)-Astaxanthin; (3S,3'S)-all-trans-Astaxanthin; (S,S)-Astaxanthin; Astaxanthin, all-trans-; all-trans-Astaxanthin; trans-Astaxanthin [1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.776 |
| E number | E161j (colours) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C40H52O4 | |
| Molar mass | 596.84 g/mol |
| Appearance | red solid powder |
| Density | 1.071 g/mL [2] |
| Melting point | 216 °C (421 °F; 489 K)[2] |
| Boiling point | 774 °C (1,425 °F; 1,047 K)[2] |
| Solubility | 30 g/L in DCM; 10 g/L in CHCl3; 0.5 g/L in DMSO; 0.2 g/L in acetone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Like many carotenoids, astaxanthin is a lipid-soluble pigment. Its red-orange colour is due to the extended chain of conjugated (alternating double and single) double bonds at the centre of the compound. This chain of conjugated double bonds is also responsible for the antioxidant function of astaxanthin (as well as other carotenoids) as it results in a region of decentralized electrons that can be donated to reduce a reactive oxidizing molecule.
Astaxanthin is a blood-red pigment and is produced naturally in the freshwater microalgae Haematococcus pluvialis and the yeast fungus Xanthophyllomyces dendrorhous (also known as Phaffia). When the algae is stressed by lack of nutrients, increased salinity, or excessive sunshine, it creates astaxanthin. Animals who feed on the algae, such as salmon, red trout, red sea bream, flamingos, and crustaceans (i.e. shrimp, krill, crab, lobster, and crayfish), subsequently reflect the red-orange astaxanthin pigmentation to various degrees.
The structure of astaxanthin by synthesis was described in 1975.[5] Astaxanthin is not converted to vitamin A in the human body so it is completely nontoxic if given orally.
Astaxanthin can also be used as a dietary supplement intended for human, animal, and aquaculture consumption. The industrial production of astaxanthin comes from plant- or animal-based and synthetic sources. The U.S. Food and Drug Administration has approved astaxanthin as a food coloring (or color additive) for specific uses in animal and fish foods.[6] The European Commission considers it food dye and it is given the E number E161j.[7] Astaxanthin from algae, synthetic and bacterial sources, is generally recognized as safe (GRAS) by the FDA.[8][9] The European Food Safety Authority has set an Acceptable Daily Intake of 0.2 mg per kg body weight in 2019. [10] As a food color additive astaxanthin and astaxanthin dimethyldisuccinate are restricted for use in Salmonid fish feed only.[11]
Natural sources

.jpg.webp)
Astaxanthin is present in most red-coloured aquatic organisms. The content varies from species to species, but also from individual to individual as it is highly dependent on diet and living conditions. Astaxanthin, and other chemically related asta-carotenoids, has also been found in a number of lichen species of the arctic zone.
The primary natural sources for industrial production of astaxanthin comprise the following:
- Euphausia pacifica (Pacific krill)
- Euphausia superba (Antarctic krill)
- Haematococcus pluvialis (algae)[3]
- Pandalus borealis (Arctic shrimp)
- Xanthophyllomyces dendrorhous, formerly Phaffia rhodozyma (yeast)
Astaxanthin concentrations in nature are approximately:
| Source | Astaxanthin concentration (ppm) |
|---|---|
| Salmonids | ~ 5 |
| Plankton | ~ 60 |
| Krill | ~ 120 |
| Arctic shrimp (P borealis) | ~ 1,200 |
| Phaffia yeast | ~ 10,000 |
| Haematococcus pluvialis | ~ 40,000 |
Algae are the primary natural source of astaxanthin in the aquatic food chain. The microalgae Haematococcus pluvialis seems to accumulate the highest levels of astaxanthin in nature and is currently, the primary industrial source for natural astaxanthin production where more than 40 g of astaxanthin can be obtained from one kg of dry biomass. Haematococcus pluvialis has the productional advantage of the population doubling every week, which means scaling up is not an issue. Specifically, the microalgae are grown in two phases. First, in the green phase, the cells are given an abundance of nutrients to promote proliferation of the cells. In the subsequent red phase, the cells are deprived of nutrients and subjected to intense sunlight to induce encystment (carotogenesis), during which the cells produce high levels of astaxanthin as a protective mechanism against the environmental stress. The cells, with their high concentrations of astaxanthin, are then harvested.[12]
Phaffia yeast Xanthophyllomyces dendrorhous exhibits 100% free, non-esterified astaxanthin, which is considered advantageous because it is readily absorbable and need not be hydrolysed in the digestive tract of the fish. In contrast to synthetic and bacteria sources of astaxanthin, yeast sources of astaxanthin consist mainly of the (3R, 3’R)-form, an important astaxanthin source in nature. Finally, the geometrical isomer, all-E, is higher in yeast sources of astaxanthin, as compared to synthetic sources.
In shellfish, astaxanthin is almost exclusively concentrated in the shells, with only low amounts in the flesh itself, and most of it only becomes visible during cooking as the pigment separates from the denatured proteins that otherwise bind it. Astaxanthin is extracted from Euphausia superba (Antarctic krill)[13] and from shrimp processing waste. 12,000 pounds of wet shrimp shells can yield a 6–8 gallon astaxanthin/triglyceride oil mixture.[14]
Biosynthesis
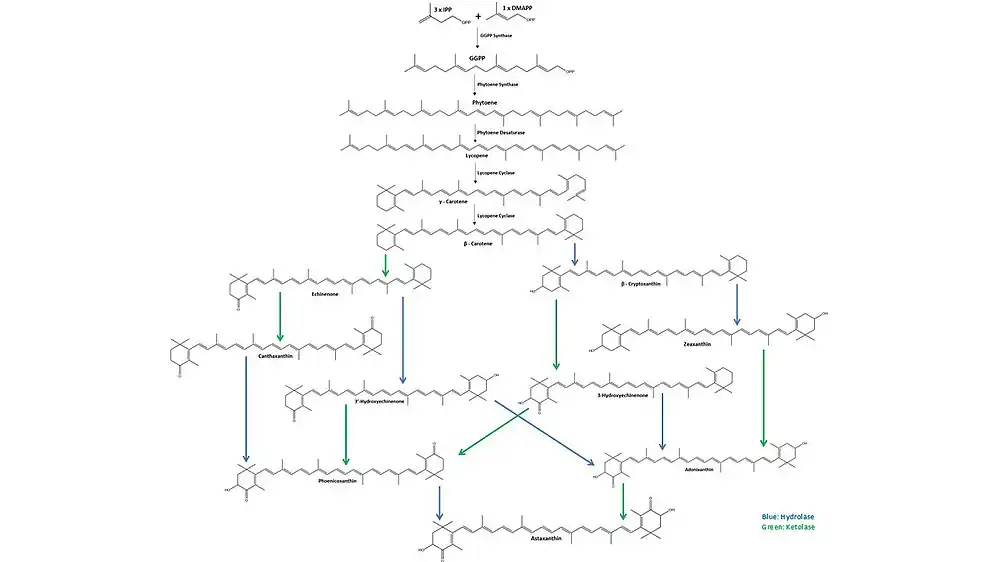
Astaxanthin biosynthesis starts with three molecules of isopentenyl pyrophosphate (IPP) and one molecule of dimethylallyl pyrophosphate (DMAPP) that are combined by IPP isomerase and converted to geranylgeranyl pyrophosphate (GGPP) by GGPP synthase. Two molecules of GGPP are then coupled by phytoene synthase to form phytoene. Next, phytoene desaturase creates four double bonds in the phytoene molecule to form lycopene. After desaturation, lycopene cyclase first forms γ-carotene by converting one of the ψ acyclic ends of the lycopene as a β-ring, then subsequently converts the other to form β-carotene. From β-carotene, hydrolases (blue) are responsible for the inclusion of two 3-hydroxy groups, and ketolases (green) for the addition of two 4-keto groups, forming multiple intermediate molecules until the final molecule, astaxanthin, is obtained.[15]
Synthetic sources
Nearly all commercially available astaxanthin for aquaculture is produced synthetically, with an annual turnover of over $200 million and a selling price of roughly $5000–6000 per kilo as of July 2012. The market grew to over $500 million by 2016 and is expected to continue to grow with the aquaculture industry.[16]
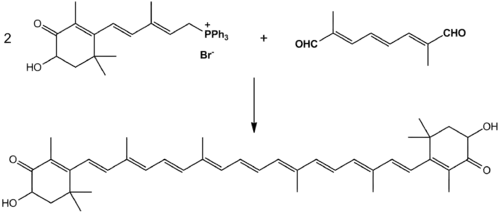
An efficient synthesis from isophorone, cis-3-methyl-2-penten-4-yn-1-ol and a symmetrical C10-dialdehyde has been discovered and is used in industrial production. It combines these chemicals together with an ethynylation and then a Wittig reaction.[17] Two equivalents of the proper ylide combined with the proper dialdehyde in a solvent of methanol, ethanol, or a mixture of the two, yields astaxanthin in up to 88% yields.[18]
Metabolic engineering
The cost of astaxanthin production, high market price and lack of a leading fermentation production systems, combined with the intricacies of chemical synthesis mean that research into alternative fermentation production methods has been carried out. Metabolic engineering offers the opportunity to create biological systems for the production of a specific target compound. The metabolic engineering of bacteria (Escherichia coli) recently allowed production of astaxanthin at >90% of the total carotenoids, providing the first engineered production system capable of efficient astaxanthin production.[19] Astaxanthin biosynthesis proceeds from beta-carotene via either zeaxanthin or canthaxanthin. Historically, it has been assumed that astaxanthin biosynthesis proceeds along both routes. However, recent work has suggested that efficient biosynthesis may, in fact, proceed from beta-carotene to astaxanthin via zeaxanthin.[20][21] The production of astaxanthin by metabolic engineering, in isolation, will not provide a suitable alternative to current industrial methods. Rather, a bioprocess approach should be adopted. Such an approach would consider fermentation conditions and economics, as well as downstream processing (extraction). Carotenoid extraction has been studied extensively, for example, the extraction of canthaxanthin (a precursor to astaxanthin) was studied within an E. coli production process demonstrating that extraction efficiency was increased substantially when two solvents, acetone and methanol, were used sequentially rather than as a combined solution.[22]
Structure
Stereoisomers
In addition to structural isomeric configurations, astaxanthin also contains two chiral centers at the 3- and 3′-positions, resulting in three unique stereoisomers (3R,3′R and 3R,3′S meso and 3S,3′S). While all three stereoisomers are present in nature, relative distribution varies considerably from one organism to another.[23] Synthetic astaxanthin contains a mixture of all three stereoisomers, in approximately 1:2:1 proportions.
Esterification
Astaxanthin exists in two predominant forms, non-esterified (yeast, synthetic) or esterified (algal) with various length fatty acid moieties whose composition is influenced by the source organism as well as growth conditions. The astaxanthin fed to salmon to enhance flesh coloration is in the non-esterified form [24] The predominance of evidence supports a de-esterification of fatty acids from the astaxanthin molecule in the intestine prior to or concomitant with absorption resulting in the circulation and tissue deposition of non-esterified astaxanthin. European Food Safety Authority (EFSA) published a scientific opinion on a similar xanthophyll carotenoid, lutein, stating that “following passage through the gastrointestinal tract and/or uptake lutein esters are hydrolyzed to form free lutein again”.[25] While it can be assumed that non-esterified astaxanthin would be more bioavailable than esterified astaxanthin due to the extra enzymatic steps in the intestine needed to hydrolysis the fatty acid components, several studies suggest that bioavailability is more dependent on formulation than configuration.[26][27]
Uses
Astaxanthin is used as a dietary supplement and feed supplement as food colorant for salmon, crabs, shrimp, chickens and egg production.[28][29]
For seafood and animals
The primary use of synthetic astaxanthin today is as an animal feed additive to impart coloration, including farm-raised salmon and chicken egg yolks.[30] Synthetic carotenoid pigments colored yellow, red or orange represent about 15–25% of the cost of production of commercial salmon feed.[31] Today, almost all commercial astaxanthin for aquaculture is produced synthetically.[32]
Class action lawsuits were filed against some major grocery store chains for not clearly labeling the astaxanthin-treated salmon as "color added".[33] The chains followed up quickly by labeling all such salmon as "color added". Litigation persisted with the suit for damages, but a Seattle judge dismissed the case, ruling that enforcement of the applicable food laws was up to government and not individuals.[34]
Dietary supplement
The primary human application for astaxanthin is as a dietary supplement, although as of 2018, there was insufficient evidence from medical research to show that it affects disease risk or health of people, and it remains under preliminary research. In 2018, the European Food Safety Authority sought scientific information from manufacturers of dietary supplements about the safety of astaxanthin.[35]
Role in the food chain
Lobsters, shrimp, and some crabs turn red when cooked because the astaxanthin, which was bound to the protein in the shell, becomes free as the protein denatures and unwinds. The freed pigment is thus available to absorb light and produce the red color.[36]
Regulations
In April 2009, the United States Food and Drug Administration approved astaxanthin as an additive for fish feed only as a component of a stabilized color additive mixture. Color additive mixtures for fish feed made with astaxanthin may contain only those diluents that are suitable.[6] The color additives astaxanthin, ultramarine blue, canthaxanthin, synthetic iron oxide, dried algae meal, Tagetes meal and extract, and corn endosperm oil are approved for specific uses in animal foods.[37] Haematococcus algae meal (21 CFR 73.185) and Phaffia yeast (21 CFR 73.355) for use in fish feed to color salmonoids were added in 2000.[38][39][40] In the European Union, astaxanthin-containing food supplements derived from sources that have no history of use as a source of food in Europe, fall under the remit of the Novel Food legislation, EC (No.) 258/97. Since 1997, there have been five novel food applications concerning products that contain astaxanthin extracted from these novel sources. In each case, these applications have been simplified or substantial equivalence applications, because astaxanthin is recognised as a food component in the EU diet.[41][42][43][44]
References
- SciFinder Web (accessed Sep 28, 2010). Astaxanthin (472-61-7) Name
- SciFinder Web (accessed Sep 28, 2010). Astaxanthin (472-61-7) Experimental Properties.
- Margalith, P. Z. (1999). "Production of ketocarotenoids by microalgae". Applied Microbiology and Biotechnology. 51 (4): 431–8. doi:10.1007/s002530051413. PMID 10341427.
- Choi, Seyoung; Koo, Sangho (2005). "Efficient Syntheses of the Keto-carotenoids Canthaxanthin, Astaxanthin, and Astacene". The Journal of Organic Chemistry. 70 (8): 3328–31. doi:10.1021/jo050101l. PMID 15823009.
- Cooper, R. D. G.; Davis, J. B.; Leftwick, A. P.; Price, C.; Weedon, B. (1975). "Carotenoids and related compounds. XXXII. Synthesis of astaxanthin, hoenicoxanthin, hydroxyechinenone, and the corresponding diosphenols". J. Chem. Soc. Perkin Trans. 1 (21): 2195–2204. doi:10.1039/p19750002195.
- "Summary of Color Additives for Use in United States in Foods, Drugs, Cosmetics, and Medical Devices". See Note 1.
- E-numbers : E100- E200 Food Colours. Food-Info.net. Retrieved on 2013-04-25.
- Astaxanthin wins full GRAS status. Nutraingredients-usa.com. Retrieved on 2013-04-25.
- Algatechnologies gets GRAS for AstaPure astaxanthin. Foodnavigator-usa.com. Retrieved on 2013-04-25.
- Safety and efficacy of astaxanthin-dimethyldisuccinate (Carophyll®Stay-Pink 10%-CWS) for salmonids, crustaceans and other fish European Food Safety Authority. Retrieved on 2020-08-24.
- Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Fda.gov. Retrieved on 2019-01-16.
- Boussiba; Sammy, V.; Avigad, C.; et al. (2000) Procedure for large-scale production of astaxanthin from haematococcus. U. S. Patent 6,022,701.
- Katevas, Dimitri Sclabos (6 October 2003). The Krill. aquafeed.com
- Anderson, Lyle K. Extraction of Carotenoid Pigment from Shrimp Processing Waste. U.S. Patent 3906112. Sep 16, 1975
- Barredo, Jose; García-Estrada, Carlos; Kosalkova, Katarina; Barreiro, Carlos (2017-07-30). "Biosynthesis of Astaxanthin as a Main Carotenoid in the Heterobasidiomycetous Yeast Xanthophyllomyces dendrorhous". Journal of Fungi. 3 (3): 44. doi:10.3390/jof3030044. ISSN 2309-608X. PMC 5715937. PMID 29371561.
- "Astaxanthin Market Analysis By Source". Grand View Research. July 2017. Retrieved 20 October 2017.
- Ashford's Dictionary of Industrial Chemicals, 3rd Edition, 2011, p. 984, ISBN 095226742X.
- Krause, Wolfgang; Henrich, Klaus; Paust, Joachim; et al. Preaparation of Astaxanthin. DE 19509955. 9 March 18, 1995
- Scaife, M. A.; Burja, A. M.; Wright, P. C. (2009). "Characterization of cyanobacterial β-carotene ketolase and hydroxylase genes inEscherichia coli, and their application for astaxanthin biosynthesis". Biotechnology and Bioengineering. 103 (5): 944–955. doi:10.1002/bit.22330. PMID 19365869.
- Scaife, MA; Ma, CA; Ninlayarn, T; Wright, PC; Armenta, RE (May 22, 2012). "Comparative Analysis of β-Carotene Hydroxylase Genes for Astaxanthin Biosynthesis". Journal of Natural Products. 75 (6): 1117–24. doi:10.1021/np300136t. PMID 22616944.
- Lemuth, K; Steuer, K; Albermann, C (Apr 26, 2011). "Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin". Microbial Cell Factories. 10: 29. doi:10.1186/1475-2859-10-29. PMC 3111352. PMID 21521516.
- Scaife, M.A.; Ma, C.A.; Armenta, R.E. (May 2012). "Efficient extraction of canthaxanthin from Escherichia coli by a 2-step process with organic solvents". Bioresource Technology. 111: 276–281. doi:10.1016/j.biortech.2012.01.155. PMID 22353211.
- Bjerkeng, Bjørn (1997). "Chromatographic Analysis of Synthesized Astaxanthin—A Handy Tool for the Ecologist and the Forensic Chemist?". The Progressive Fish-Culturist. 59 (2): 129–140. doi:10.1577/1548-8640(1997)059<0129:caosaa>2.3.co;2. ISSN 0033-0779.
- Rüfer, Corinna E.; Moeseneder, Jutta; Briviba, Karlis; Rechkemmer, Gerhard; Bub, Achim (2008). "Bioavailability of astaxanthin stereoisomers from wild (Oncorhynchus spp.) and aquacultured (Salmo salar) salmon in healthy men: a randomised, double-blind study". The British Journal of Nutrition. 99 (5): 1048–54. doi:10.1017/s0007114507845521. ISSN 0007-1145. PMID 17967218.
- "Scientific Opinion on the re-evaluation of lutein preparations other than lutein with high concentrations of total saponified carotenoids at levels of at least 80%". EFSA Journal. 9 (5): 2144. 2011. doi:10.2903/j.efsa.2011.2144. ISSN 1831-4732.
- Landrum, J; Bone, R; Mendez, V; Valenciaga, A; Babino, D (2012). "Comparison of dietary supplementation with lutein diacetate and lutein: a pilot study of the effects on serum and macular pigment". Acta Biochimica Polonica. 59 (1): 167–9. doi:10.18388/abp.2012_2198. ISSN 0001-527X. PMID 22428144.
- Norkus, EP; Norkus, KL; Dharmarajan, TS; Schierle, J; Schalch, W (2010). "Serum lutein response is greater from free lutein than from esterified lutein during 4 weeks of supplementation in healthy adults". Journal of the American College of Nutrition. 29 (6): 575–85. doi:10.1080/07315724.2010.10719896. ISSN 0731-5724. PMID 21677121.
- Astaxanthin and Health and Wellness in Animals. astaxanthin.org
- Ambati, Ranga Rao; Phang, Siew-Moi; Ravi, Sarada; Aswathanarayana, Ravishankar Gokare (2014-01-07). "Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review". Marine Drugs. 12 (1): 128–152. doi:10.3390/md12010128. PMC 3917265. PMID 24402174.
- Shah, M. M; Liang, Y; Cheng, J. J; Daroch, M (2016). "Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products". Frontiers in Plant Science. 7: 531. doi:10.3389/fpls.2016.00531. PMC 4848535. PMID 27200009.
- Fisheries and Oceans Canada — Aquaculture Issues. pac.dfo-mpo.gc.ca.
- Juan F Martín, Eduardo Gudiña and José L Barredo (February 20, 2008). "Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein?". Microbial Cell Factories. 7: 3. doi:10.1186/1475-2859-7-3. PMC 2288588. PMID 18289382.
- "Smith & Lowney — Farm-raised Salmon Coloring". 2003. Retrieved 14 Oct 2009. (registration required)
- "Pigments in Salmon Aquaculture: How to Grow a Salmon-colored Salmon". Archived from the original on 13 October 2007. Retrieved 18 July 2009.
- "Call for data relevant to the safety assessment of Astaxanthin in the framework of Regulation 2283/2015". European Food Safety Authority. 25 July 2018. Retrieved 16 January 2019.
- Why do crabs, lobsters & shrimp turn red when cooked?. Ochef.com. Retrieved on 2013-04-25.
- See 21 CFR 73.35,73.50, 73.75, 73.200, 73.275, 73.295, 73.315, respectively.
- Code of Federal Regulations Title 21 §73.35 FDA decision on Astaxanthin. Accessdata.fda.gov. Retrieved on 2013-04-25.
- Code of Federal Regulations Title 21 §73.185 FDA decision on Haematococcus algae meal. Accessdata.fda.gov. Retrieved on 2013-04-25.
- Food Additive Status List. fda.gov}
- Astaxanthin extract. acnfp.food.gov.uk
- Astaxanthin extract: Cyanotech Corporation. acnfp.gov.uk
- Astaxanthin extract: Algatechnologies (1998) Ltd. acnfp.gov.uk
- Astaxanthin extract: Parry Nutraceuticals. acnfp.gov.uk