Cadmium acetate
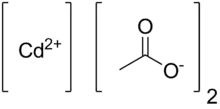
Cadmium acetate is the chemical compound with the formula Cd(CH3CO2)2. This colourless solid is classified coordination polymer, featuring acetate ligands interconnecting cadmium centers. The compound exists in anhydrous form and as a dihydrate. It forms by treating cadmium oxide with acetic acid:[3][4]
 | |
| Names | |
|---|---|
| IUPAC name
Cadmium acetate | |
| Other names
Cadmium diacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.049 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2570 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cd(CH3COO)2 (anhydrous) Cd(CH3COO)2·2H2O (dihydrate) | |
| Molar mass | 230.500 g/mol (anhydrous) 266.529 g/mol (dihydrate) |
| Appearance | colorless crystals (anhydrous) white crystals (dihydrate) |
| Odor | acetic acid |
| Density | 2.341 g/cm3 (anhydrous) 2.01 g/cm3 (dihydrate) |
| Melting point | 255 °C (491 °F; 528 K) (anhydrous) dihydrate decomposes at 130°C [1] |
| soluble (anhydrous), very soluble (dihydrate) | |
| Solubility | soluble in methanol, ethanol (anhydrous) soluble in ethanol (dihydrate) |
| -83.7·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H302, H312, H332, H400, H410 | |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P391, P501 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1027] TWA 0.005 mg/m3 (as Cd)[2] |
REL (Recommended) |
Ca[2] |
IDLH (Immediate danger) |
Ca [9 mg/m3 (as Cd)][2] |
| Related compounds | |
Other anions |
Cadmium fluoride Cadmium chloride Cadmium bromide Cadmium iodide |
Other cations |
Zinc acetate Mercury(II) acetate Silver acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Cadmium acetate is used for glazing ceramics and pottery; in electroplating baths, in dyeing and printing textiles; and as an analytic reagent for sulfur, selenium and tellurium.[4]
Preparation
Cadmium acetate is prepared by treating cadmium oxide with acetic acid. The compound may also be prepared by treating cadmium nitrate with acetic anhydride.
Safety
Cadmium compounds are considered Group 1 carcinogens by the IARC.
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 447. ISBN 0-8493-0594-2.
- NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- Gangolli, S. (1999). The Dictionary of Substances and Their Effects. London: Royal Society of Chemistry. pp. 12–13. Retrieved 2009-03-29.
- Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill Professional. pp. 143–144. ISBN 0-07-049439-8. Retrieved 2009-03-29.
| AcOH | He | |||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | |||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | |||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | |||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | |||
| CsOAc | Ba(OAc)2 | * | Lu(OAc)3 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | ||
| Fr | Ra | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||
| * | La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | ||||||
| ** | Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | ||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
