Cadmium tetrafluoroborate
Cadmium tetrafluoroborate is an ionic, chemical compound with the formula Cd(BF4)2.[3] It is an ionic, crystalline solid, which is colorless and odorless. Cadmium tetrafluoroborate is most frequently used in the industrial production of high-strength steels, its purpose being to prevent hydrogen absorption, a source of post-production cracking of the metal, in the treated steels. Another application of the chemistry of cadmium tetrafluoroborate is fine tuning of the size of cadmium telluride nanomaterials.
 | |
| Names | |
|---|---|
| Other names
Cadmium(II) tetrafluoroborate
Cadmium fluoroborate Cadmium fluoborate | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.034.975 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cd(BF4)2 | |
| Molar mass | 286.020 g/mol |
| Appearance | colorless solid crystals very hygroscopic |
| Odor | odorless |
| Density | 1.60 g/cm3[1] |
| very soluble | |
| Solubility | very soluble in alcohol |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1027] TWA 0.005 mg/m3 (as Cd)[2] |
REL (Recommended) |
Ca[2] |
IDLH (Immediate danger) |
Ca [9 mg/m3 (as Cd)][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
While the use of cadmium tetrafluoroborate is limited, concerns about limited or chronic exposure to this substance should be brought to the attention of a physician or other trained medical staff. Exposure to cadmium tetrafluoroborate, via ingestion, contact with the skin or mucous membranes, or inhalation can have lasting and harmful health effects.
Preparation
Cadmium tetrafluoroborate may be prepared from the reaction between an aqueous solution of fluoroboric acid and cadmium carbonate or cadmium oxide:[4]
It is also possible to prepare cadmium tetrafluoroborate through an oxidation-reduction reaction implementing nitrosyl tetrafluoroborate:[5]
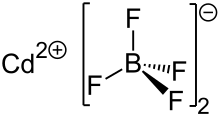
Structure
Cadmium tetrafluoroborate
Cadmium tetrafluoroborate is an ionic compound formed from the two, ionic species Cd2+ and BF4−. At room temperature it forms colorless, odorless crystals which are soluble in polar solvents such as water or ethanol. At room temperature, the hydrated salt, Cd(BF4)2·6H2O, exists in a monoclinic crystal system, though this is temperature dependent.[6] Two, first-order phase transitions have been noted in the literature for this material, one each at 324 K and 177 K, representing a change in the crystal system from monoclinic to trigonal and from trigonal to either monoclinic or triclinic, respectively.[6] The quasi-trigonal geometry of the cadmium tetrafluoroborate hexahydrate crystal is unique among the first-row transition metal tetrafluoroborates and perchlorates, which have predominately hexagonal structures.[7]
Related transition-metal complexes
The Cd2+ species of cadmium tetrafluoroborate may associate with various ligands to form transition-metal complexes. The structural formulas and geometries for such complexes can vary depending upon coordination number of the complex and the electronic properties of the ligands (see also, HSAB theory). However, two general forms may predominate: (i) [Cd(L)n(BF4)m], where L and BF4− are ligands in the inner-sphere, and (ii) [Cd(L)n](BF4)2, where BF4− is located in the outer-sphere;[8] for both, n=1,2,…,6. The literature contains reports of distorted octahedral geometries[9][10] for Cadmium tetrafluoroborate complexes with nitrogen-containing ligands such as pyrazoles and imidazoles[9] and porphyrins.[10] Given the structural formulas for Cadmium tetrafluoroborate complexes noted in the literature[8] however, such as [Cd(L)4(BF4)2], it is likely that tetrahedral geometries are also possible in such complexes.
Uses
Electroplating
The most significant, industrial use of Cd(BF4)2 is in the electroplating of high-strength steels.[11] Here, species such as cadmium tetrafluoroborate (or Cd-Ti or CdCN) are deposited on the surface of steels in an electroplating process which inhibits absorption of hydrogen onto the surface of the steels, a source of cracking following baking of the metal.[11] Optimization of the electroplating process, adjusting electrolyte concentrations in Cadmium tetrafluoroborate mixes, has been explored in the literature.[12] Among other methods of electroplating, cadmium tetrafluoroborate baths have middling efficiency. It has, for instance, been demonstrated that traditional cyanide bathes (e.g. CdCN or ZnCN) and variants there-of provide more efficient distribution of current density during electroplating, resulting in steels which could bear greater loads.[13]
Nanomaterials
A method of etching of CdTe nanocrystals which removes Cd from the surface of the nano-structures via attack by tetrafluoroborate anions has been reported in the literature.[14] While the presence of Cd-F surface bonds and dissociation of Cd from the surface of the nano-structures are clear from the investigation, complex formation of Cd with BF4− in solution was not discussed though may be inferred from the spectrophotometric results.[14]
Determination of boron in steels by solvent extraction
Methodology has been reported for the determination of boron concentration in steels using cadmium tetrafluoroborate complex formation during solvent extraction to facilitate indirect atomic absorption measurements.[15] Tetrafluoroborate, formed from acid extraction of boron for a steel sample using boric acid, associates with a transition metal complex of Cd2+ and forms a complex which is measureable by atomic absorption spectroscopy.[15] Similar procedures have been implemented for the same purpose using other transition metals[16] and for determination of boron in high-purity silicon using other cadmium tetrafluoroborate transition metal complexes.[17]
Hazards and Safety
Biological hazards, safety, and treatment
Cadmium tetreafluoroborate is a caustic substance, particularly when in aqueous solution. Multiple routes of exposure, such as ingestion, inhalation, or contact with the skin or mucous membranes, are available through contact with aqueous cadmium tetrafluorobromate.[18][19] Target biological systems following exposure include the lungs, kidneys, and liver.[18][19] Symptoms of cadmium tetrafluoroborate exposure include nausea, vomiting, fever, irritation of the mucous membranes (e.g. upper respiratory tract, eyes) and skin, coughing, wheezing, or difficulty breathing.[18] The mechanism of toxicity of this substance is related to cadmium poisoning and exposure to borates and hydrofluoric acid.[19] The compound functions in solution as a weakly acidic inorganic salt, neutralizing bases.[20] After initial exposure, thorough rising of the affected area with water is recommended. However, seeking medical attention is strongly advised as treatment for exposure to Cd or F containing compounds such as cadmium tetrafluoroborate generally involves intravenous administration (I.V.) of calcium chloride and sodium bicarbonate for the purpose of maintaining blood pH and sequestering Cd2+ and BF4− in insoluble salts.[19]
Chronic exposure
Chronic exposure to this substance may have negative health consequences. According to its OSHA, IARC, and ACGIH ratings, cadmium tetrafluoroborate is recognized as a carcinogenic substance.[11][19][21][22] Further effects of chronic exposure may include hypocalcaemia and edemas of the respiratory system.[18]
Non-biological hazards and safety
Although this compound is a negligible fire hazard,[23] combustion of cadmium tetrafluoroborate produces hazardous decomposition products including cadmium/cadmium oxide and hydrogen fluoride. Therefore, cadmium tetrafluoroborate is stored out of direct light, in a cool environment, and away from other flammable materials.
References
- Lide, David R. (2007–2008). CRC Handbook of Chemistry and Physics, 88th Edition. Boca Raton, FL: Taylor & Francis. ISBN 9780849304880.
- NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- Hazardous Substances Database. toxnet.nlm.nih.gov
- "Compound Summary for PubChem CID 12886773". NIH. 2017.
- Hathaway, B. J.; Holah, D. G.; Underhill, A. E. (1962). "The preparation and properties of some bivalent transition-metal tetrafluoroborate–methyl cyanide complexes". Journal of the Chemical Society: 2444–8. doi:10.1039/JR9620002444.
- Jain, A.K.; Geoffroy, M. (October 1981). "ESR study of phase transitions in single crystals of Cd(BF4)2, 6H2O". Solid State Communications. 40 (1): 33–35. Bibcode:1981SSCom..40...33J. doi:10.1016/0038-1098(81)90705-5.
- Moss, K.C.; Russell, D.R.; Sharp, D.W.A. (1961). "The lattice constants of some metal-fluoroborate hexahydrates". Acta Crystallographica. 14 (3): 330. doi:10.1107/S0365110X61001133.
- Chebotarev, A. N.; Shestakova, M. V.; Kuz'min, V. E.; Artemenko, A. G. (2005-09-01). "The Maximum Hardness Principle and the Composition of Zn(II) and Cd(II) Tetrafluoroborate Complexes with Nitrogen-Containing Organic Bases". Russian Journal of Coordination Chemistry. 31 (9): 619–622. doi:10.1007/s11173-005-0145-8. S2CID 93456957.
- J., Reedijk; G.C., Verschoor (1973-04-15). "Pyrazoles and imidazoles as ligands. XX. The crystal and molecular structure of hexakis(2-methylimidazole)cadmium(II) tetrafluoroborate" (PDF). Acta Crystallographica B. 29 (4): 721. doi:10.1107/S0567740873003237.
- Tomat, Elisa; Cuesta, Luciano; Lynch, Vincent M.; Sessler, Jonathan L. (2007-08-01). "Binuclear Fluoro-Bridged Zinc and Cadmium Complexes of a Schiff Base Expanded Porphyrin: Fluoride Abstraction from the Tetrafluoroborate Anion". Inorganic Chemistry. 46 (16): 6224–6226. doi:10.1021/ic700933p. PMID 17630733.
- Dini, J. W. (1993). Electrodeposition: The Materials Science of Coatings and Substrates. Noyes Publications. ISBN 9780815513209.
- Zagurskii, I. N.; Ozerov, A. M. (1973). "Cadmium plating of steel wire in hydrogen tetrafluoroborate electrolytes". Zashchita Metallov. Volgograd. INst. INzh. Gor. Khoz. 9 (2): 221–3.
- Geyer, N. M.; Lawless, G. W.; Cohen, B. (1958-11-14). "A New Look at the Hydrogen Embrittlement of Cadmium Coated High Strength Steels. Period Covered: January 1956 to May 1958". OSTI 4286738. Cite journal requires
|journal=(help) - Liu, Jianbo; Yang, Xiaohai; Wang, Kemin; Wang, Dong; Zhang, Pengfei (2009-10-06). "Chemical etching with tetrafluoroborate: a facile method for resizing of CdTe nanocrystals under mild conditions". Chemical Communications (40): 6080–2. doi:10.1039/B910752E. PMID 19809650.
- Hayashi, Y.; Matsushita, S.; Kumamaru, T.; Yamamoto, Y. (April 1973). "Indirect atomic-absorption determination of boron by solvent extraction as tris(1,10-phenanthroline)cadmium tetrafluoroborate". Talanta. 20 (4): 414–416. doi:10.1016/0039-9140(73)80171-7. PMID 18961297.
- Donaldson, E. (November 1981). "Spectrophotometric determination of boron in iron and steel with curcumin after separation by 2-ethyl-1,3-hexanediol-chloroform extraction". Talanta. 28 (11): 825–831. doi:10.1016/0039-9140(81)80024-0. PMID 18963014.
- Liu, C. Y.; Chen, P. Y.; Lin, H. M.; Yang, M. H. (1985-01-01). "Determination of boron in high-purity silicon and trichlorosilane indirectly by measurement of cadmium in tris (1,10-phenanthroline) cadmium tetrafluoroborate". Fresenius' Zeitschrift für analytische Chemie. 320 (1): 22–28. doi:10.1007/BF00481073. S2CID 91528317.
- "Cadmium tetrafluoroborate solution 481734". Sigma-Aldrich. Retrieved 2017-05-05.
- "TOXNET". toxnet.nlm.nih.gov. Retrieved 2017-05-05.
- GOV, NOAA Office of Response and Restoration, US. "CADMIUM FLUOROBORATE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2017-05-05.
- Chambers, Michael. "ChemIDplus – 14486-19-2 – NXOFSPIMFJTFSE-UHFFFAOYSA-N – Cadmium fluoborate – Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.nlm.nih.gov. Retrieved 2017-05-05.
- "IARC Monographs- Classifications". monographs.iarc.fr. Retrieved 2017-05-05.
- "Material Safety Data Sheet: Cadmium Fluoborate" (PDF). BassTech International. 2014.