Chimera (genetics)
A genetic chimerism or chimera (/kaɪˈmɪərə/ ky-MEER-ə or /kɪˈmɪərə/ kə-MEER-ə, also spelled chimaera or chimæra) is a single organism composed of cells with more than one distinct genotype. In animals, this means an individual derived from two or more zygotes, which can include possessing blood cells of different blood types, subtle variations in form (phenotype) and, if the zygotes were of differing sexes, then even the possession of both female and male sex organs[1] (this is just one of many different phenomena that may result in intersexuality). Animal chimeras are produced by the merger of multiple fertilized eggs. In plant chimeras, however, the distinct types of tissue may originate from the same zygote, and the difference is often due to mutation during ordinary cell division. Normally, genetic chimerism is not visible on casual inspection; however, it has been detected in the course of proving parentage.[2]

Another way that chimerism can occur in animals is by organ transplantation, giving one individual tissues that developed from a different genome. For example, transplantation of bone marrow often determines the recipient's ensuing blood type.
Animals
An animal chimera is a single organism that is composed of two or more different populations of genetically distinct cells that originated from different zygotes involved in sexual reproduction. If the different cells have emerged from the same zygote, the organism is called a mosaic. Chimeras are formed from at least four parent cells (two fertilised eggs or early embryos fused together). Each population of cells keeps its own character and the resulting organism is a mixture of tissues. Cases of human chimerism have been documented.[1]
This condition is either inherited or it is acquired through the infusion of allogeneic hematopoietic cells during transplantation or transfusion. In nonidentical twins, chimerism occurs by means of blood-vessel anastomoses. The likelihood of offspring being a chimera is increased if it is created via in vitro fertilisation.[3] Chimeras can often breed, but the fertility and type of offspring depends on which cell line gave rise to the ovaries or testes; varying degrees of intersex differences may result if one set of cells is genetically female and another genetically male.
Tetragametic chimerism

Tetragametic chimerism is a form of congenital chimerism. This condition occurs through the fertilization of two separate ova by two sperm, followed by aggregation of the two at the blastocyst or zygote stages. This results in the development of an organism with intermingled cell lines. Put another way, the chimera is formed from the merging of two nonidentical twins (a similar merging presumably occurs with identical twins, but as their genotypes are not significantly distinct, the resulting individual would not be considered a chimera). As such, they can be male, female, or have mixed intersex characteristics.[4][5][6][7][8][3][9]
As the organism develops, it can come to possess organs that have different sets of chromosomes. For example, the chimera may have a liver composed of cells with one set of chromosomes and have a kidney composed of cells with a second set of chromosomes. This has occurred in humans, and at one time was thought to be extremely rare although more recent evidence suggests that this is not the case.[1][10]
This is particularly true for the marmoset. Recent research shows most marmosets are chimeras, sharing DNA with their fraternal twins.[11] 95% of marmoset fraternal twins trade blood through chorionic fusions, making them hematopoietic chimeras.[12][13]
Most chimeras will go through life without realizing they are chimeras. The difference in phenotypes may be subtle (e.g., having a hitchhiker's thumb and a straight thumb, eyes of slightly different colors, differential hair growth on opposite sides of the body, etc.) or completely undetectable. Chimeras may also show, under a certain spectrum of UV light, distinctive marks on the back resembling that of arrow points pointing downwards from the shoulders down to the lower back; this is one expression of pigment unevenness called Blaschko's lines.[14]
Affected persons may be identified by the finding of two populations of red cells or, if the zygotes are of opposite sex, ambiguous genitalia and intersex alone or in combination; such persons sometimes also have patchy skin, hair, or eye pigmentation (heterochromia). If the blastocysts are of opposite sex, genitals of both sexes may be formed: either ovary and testis, or combined ovotestes, in one rare form of intersex, a condition previously known as true hermaphroditism.
Note that the frequency of this condition does not indicate the true prevalence of chimerism. Most chimeras composed of both male and female cells probably do not have an intersex condition, as might be expected if the two cell populations were evenly blended throughout the body. Often, most or all of the cells of a single cell type will be composed of a single cell line, i.e. the blood may be composed predominantly of one cell line, and the internal organs of the other cell line. Genitalia produce the hormones responsible for other sex characteristics.
Natural chimeras are almost never detected unless they exhibit abnormalities such as male/female or hermaphrodite characteristics or uneven skin pigmentation. The most noticeable are some male tortoiseshell cats and calico cats (although most male tortoiseshells have an extra X chromosome responsible for the colouration) or animals with ambiguous sex organs.
The existence of chimerism is problematic for DNA testing, a fact with implications for family and criminal law. The Lydia Fairchild case, for example, was brought to court after DNA testing apparently showed that her children could not be hers. Fraud charges were filed against her and her custody of her children was challenged. The charge against her was dismissed when it became clear that Lydia was a chimera, with the matching DNA being found in her cervical tissue. Another case was that of Karen Keegan, who was also suspected (initially) of not being her children's biological mother, after DNA tests on her adult sons for a kidney transplant she needed seemed to show she was not their mother.[1][15]
The tetragametic state has important implications for organ or stem cell transplantation. Chimeras typically have immunologic tolerance to both cell lines.
Microchimerism
Microchimerism is the presence of a small number of cells that are genetically distinct from those of the host individual. Most people are born with a few cells genetically identical to their mothers' and the proportion of these cells goes down in healthy individuals as they get older. People who retain higher numbers of cells genetically identical to their mother's have been observed to have higher rates of some autoimmune diseases, presumably because the immune system is responsible for destroying these cells and a common immune defect prevents it from doing so and also causes autoimmune problems. The higher rates of autoimmune diseases due to the presence of maternally-derived cells is why in a 2010 study of a 40-year-old man with scleroderma-like disease (an autoimmune rheumatic disease), the female cells detected in his blood stream via FISH (fluorescence in situ hybridization) were thought to be maternally-derived. However, his form of microchimerism was found to be due to a vanished twin, and it is unknown whether microchimerism from a vanished twin might predispose individuals to autoimmune diseases as well.[16] Mothers often also have a few cells genetically identical to those of their children, and some people also have some cells genetically identical to those of their siblings (maternal siblings only, since these cells are passed to them because their mother retained them).
Symbiotic chimerism in anglerfish
Chimerism occurs naturally in adult Ceratioid anglerfish and is in fact a natural and essential part of their life cycle. Once the male achieves adulthood, it begins its search for a female. Using strong olfactory (or smell) receptors, the male searches until it locates a female anglerfish. The male, less than an inch in length, bites into her skin and releases an enzyme that digests the skin of both his mouth and her body, fusing the pair down to the blood-vessel level. While this attachment has become necessary for the male's survival, it will eventually consume him, as both anglerfish fuse into a single hermaphroditic individual. Sometimes in this process, more than one male will attach to a single female as a symbiote. In this case, they will all be consumed into the body of the larger female angler. Once fused to a female, the males will reach sexual maturity, developing large testicles as their other organs atrophy. This process allows for sperm to be in constant supply when the female produces an egg, so that the chimeric fish is able to have a greater number of offspring.[17]
Germline chimerism
Germline chimerism occurs when the germ cells (for example, sperm and egg cells) of an organism are not genetically identical to its own. It has been recently discovered that marmosets can carry the reproductive cells of their (fraternal) twin siblings due to placental fusion during development. (Marmosets almost always give birth to fraternal twins.)[11][18][19]
Artificial chimerism
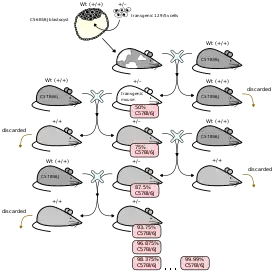
Artificial chimerism falls under the artificial category in which a chimera can exist. An individual that falls under this classification possesses two different sets of genetic pedigrees: one that was inherited genetically at the time of the formation of the human embryo and the other that was intentionally introduced through a medical procedure known as transplantation.[20] Specific types of transplants that could induce this condition include bone marrow transplants and organ transplants, as the recipient's body essentially works to permanently incorporate the new blood stem cells into it.
An example of artificial chimerism in animals are the quail-chick chimeras. By utilizing transplantation and ablation in the chick embryo stage, the neural tube and the neural crest cells of the chick were ablated, and replaced with the same parts from a quail.[21] Once hatched, the quail feathers were visibly apparent around the wing area, whereas the rest of the chick's body was made of its own chicken cells.
Humans
Chimerism has been documented in humans in several instances.
- The Dutch sprinter Foekje Dillema was expelled from the 1950 national team after she refused a mandatory sex test in July 1950; later investigations revealed a Y-chromosome in her body cells, and the analysis showed that she was probably a 46,XX/46,XY mosaic female.[22]
- In 1953 a human chimera was reported in the British Medical Journal. A woman was found to have blood containing two different blood types. Apparently this resulted from her twin brother's cells living in her body.[23] A 1996 study found that such blood group chimerism is not rare.[24]
- Another report of a human chimera was published in 1998, where a male human had some partially developed female organs due to chimerism. He had been conceived by in-vitro fertilization.[3]
- In 2002, Lydia Fairchild was denied public assistance in Washington state when DNA evidence appeared to show that she was not the mother of her children. A lawyer for the prosecution heard of a human chimera in New England, Karen Keegan, and suggested the possibility to the defense, who were able to show that Fairchild, too, was a chimera with two sets of DNA, and that one of those sets could have been the mother of the children.[25]
- In 2002, an article in the New England Journal of Medicine describes a woman in whom tetragametic chimerism was unexpectedly identified after undergoing preparations for kidney transplant that required the patient and her immediate family to undergo histocompatibility testing, the result of which suggested that she was not the biologic mother of two of her three children.[26]
- In 2009, singer Taylor Muhl discovered that what was always thought to be a large birthmark on her torso was actually caused by chimerism.
- In 2017, a human-pig chimera was reported to have been created; the chimera was also reported to have 0.001% human cells, with the balance being pig.[27][28][29]
Hermaphrodites
- Debate exists surrounding true hermaphrodites in regards to a hypothetical scenario in which it could be possible for a human to self-fertilize. If a human chimera is formed from a male and female zygote fusing into a single embryo, giving an individual functional gonadal tissue of both types, such a self-fertilization is feasible. Indeed, it is known to occur in non-human species where hermaphroditic animals are common. However, no such case of functional self-fertilization has ever been documented in humans.[30]
Bone marrow recipients
- Several cases of unusual chimera phenomena have been reported in bone marrow recipients.
- In 2019, the blood and seminal fluid of a man in Reno, Nevada (who had undergone a vasectomy), exhibited only the genetic content of his bone marrow donor. Swabs from his lips, cheek and tongue showed mixed DNA content.[31]
- The DNA content of semen from an assault case in 2004 matched that of a man who had been in prison at the time of the assault, but who had been a bone marrow donor for his brother, who was later determined to have committed the crime.[31][32][33]
- In 2008, A man was killed in a traffic accident that occurred in Seoul, South Korea. In order to identify him, his DNA was analyzed. Results revealed that the DNA of his blood, along with some of his organs, appeared to show that he was female. It was later determined that he had received a bone marrow transplant from his daughter.[31]
Chimera Identification
Chimerism is so rare, that there have only been 100 confirmed cases in humans.[34] However, this may be due to the fact that humans might not be aware that they have this condition to begin with. There are usually no signs or symptoms for chimerism other than a few physical symptoms such as hyper-pigmentation, hypo-pigmentation, or possessing two different colored eyes. However, these signs do not necessarily mean an individual is a chimera and should only be seen as possible symptoms. Again, forensic investigation or curiosity over a failed maternity/paternity DNA test usually leads to the accidental discovery of this condition. By simply undergoing a DNA test, which usually consists of either a swift cheek swab or a blood test, the discovery of the once unknown second genome is made, therefore identifying that individual as a chimera.[35]
Research
The first known primate chimeras are the rhesus monkey twins, Roku and Hex, each having six genomes. They were created by mixing cells from totipotent four cell blastocysts; although the cells never fused, they worked together to form organs. It was discovered that one of these primates, Roku, was a sexual chimera; as four percent of Roku's blood cells contained two x chromosomes.[12]
A major milestone in chimera experimentation occurred in 1984 when a chimeric sheep–goat was produced by combining embryos from a goat and a sheep, and survived to adulthood.[36]
In August 2003, researchers at the Shanghai Second Medical University in China reported that they had successfully fused human skin cells and rabbit ova to create the first human chimeric embryos. The embryos were allowed to develop for several days in a laboratory setting, and then destroyed to harvest the resulting stem cells.[37] In 2007, scientists at the University of Nevada School of Medicine created a sheep whose blood contained 15% human cells and 85% sheep cells.[38]
On January 22, 2019 the National Society of Genetic Counselors released an article — Chimerism Explained: How One Person Can Unknowingly Have Two Sets of DNA, where they state “Tetragametic Chimerism, where a twin pregnancy evolves into one child, is currently believed to be one of the rarer forms. However, we know that 20 to 30 percent of singleton pregnancies were originally a twin or a multiple pregnancy. Due to this statistic, it is quite possible that tetragametic chimerism is more common than current data implies”.[39]
Sponges
Chimerism has been found in some species of marine sponges.[40] Four distinct genotypes have been found in a single individual, and there is potential for even greater genetic heterogeneity. Each genotype functions independently in terms of reproduction, but the different intra-organism genotypes behave as a single large individual in terms of ecological responses like growth.[40]
Mice

Chimeric mice are important animals in biological research, as they allow for the investigation of a variety of biological questions in an animal that has two distinct genetic pools within it. These include insights into problems such as the tissue specific requirements of a gene, cell lineage, and cell potential. The general methods for creating chimeric mice can be summarized either by injection or aggregation of embryonic cells from different origins. The first chimeric mouse was made by Beatrice Mintz in the 1960s through the aggregation of eight-cell-stage embryos.[41] Injection on the other hand was pioneered by Richard Gardner and Ralph Brinster who injected cells into blastocysts to create chimeric mice with germ lines fully derived from injected embryonic stem cells (ES cells).[42] Chimeras can be derived from mouse embryos that have not yet implanted in the uterus as well as from implanted embryos. ES cells from the inner cell mass of an implanted blastocyst can contribute to all cell lineages of a mouse including the germ line. ES cells are a useful tool in chimeras because genes can be mutated in them through the use of homologous recombination, thus allowing gene targeting. Since this discovery occurred in 1988, ES cells have become a key tool in the generation of specific chimeric mice.[43]
Underlying biology
The ability to make mouse chimeras comes from an understanding of early mouse development. Between the stages of fertilization of the egg and the implantation of a blastocyst into the uterus, different parts of the mouse embryo retain the ability to give rise to a variety of cell lineages. Once the embryo has reached the blastocyst stage, it is composed of several parts, mainly the trophectoderm, the inner cell mass, and the primitive endoderm. Each of these parts of the blastocyst gives rise to different parts of the embryo; the inner cell mass gives rise to the embryo proper, while the trophectoderm and primitive endoderm give rise to extra embryonic structures that support growth of the embryo.[44] Two- to eight-cell-stage embryos are competent for making chimeras, since at these stages of development, the cells in the embryos are not yet committed to give rise to any particular cell lineage, and could give rise to the inner cell mass or the trophectoderm. In the case where two diploid eight-cell-stage embryos are used to make a chimera, chimerism can be later found in the epiblast, primitive endoderm, and trophectoderm of the mouse blastocyst.[45][46]
It is possible to dissect the embryo at other stages so as to accordingly give rise to one lineage of cells from an embryo selectively and not the other. For example, subsets of blastomeres can be used to give rise to chimera with specified cell lineage from one embryo. The Inner Cell Mass of a diploid blastocyst, for example, can be used to make a chimera with another blastocyst of eight-cell diploid embryo; the cells taken from the inner cell mass will give rise to the primitive endoderm and to the epiblast in the chimera mouse.[47] From this knowledge, ES cell contributions to chimeras have been developed. ES cells can be used in combination with eight-cell-and two-cell-stage embryos to make chimeras and exclusively give rise to the embryo proper. Embryos that are to be used in chimeras can be further genetically altered in order to specifically contribute to only one part of chimera. An example is the chimera built off of ES cells and tetraploid embryos, which are artificially made by electrofusion of two two-cell diploid embryos. The tetraploid embryo will exclusively give rise to the trophectoderm and primitive endoderm in the chimera.[48][49]
Methods of production
There are a variety of combinations that can give rise to a successful chimera mouse and – according to the goal of the experiment – an appropriate cell and embryo combination can be picked; they are generally but not limited to diploid embryo and ES cells, diploid embryo and diploid embryo, ES cell and tetraploid embryo, diploid embryo and tetraploid embryo, ES cells and ES cells. The combination of embryonic stem cell and diploid embryo is a common technique used for the making of chimeric mice, since gene targeting can be done in the embryonic stem cell. These kinds of chimeras can be made through either aggregation of stem cells and the diploid embryo or injection of the stem cells into the diploid embryo. If embryonic stem cells are to be used for gene targeting to make a chimera, the following procedure is common: a construct for homologous recombination for the gene targeted will be introduced into cultured mouse embryonic stem cells from the donor mouse, by way of electroporation; cells positive for the recombination event will have antibiotic resistance, provided by the insertion cassette used in the gene targeting; and be able to be positively selected for.[50][51] ES cells with the correct targeted gene are then injected into a diploid host mouse blastocyst. Then, these injected blastocysts are implanted into a pseudo pregnant female surrogate mouse, which will bring the embryos to term and give birth to a mouse whose germline is derived from the donor mouse's ES cells.[52] This same procedure can be achieved through aggregation of ES cells and diploid embryos, diploid embryos are cultured in aggregation plates in wells where single embryos can fit, to these wells ES cells are added the aggregates are cultured until a single embryo is formed and has progressed to the blastocyst stage, and can then be transferred to the surrogate mouse.[53]
Plants

Structure
The distinction between sectorial, mericlinal and periclinal plant chimeras are widely used.[54][55]
Graft chimeras

These are produced by grafting genetically different parents, different cultivars or different species (which may belong to different genera). The tissues may be partially fused together following grafting to form a single growing organism that preserves both types of tissue in a single shoot.[56] Just as the constituent species are likely to differ in a wide range of features, so the behavior of their periclinal chimeras is like to be highly variable.[57] The first such known chimera was probably the Bizzaria, which is a fusion of the Florentine citron and the sour orange. Well-known examples of a graft-chimera are Laburnocytisus 'Adamii', caused by a fusion of a Laburnum and a broom, and "Family" trees, where multiple varieties of apple or pear are grafted onto the same tree. Many fruit trees are cultivated by grafting the body of a sapling onto a rootstock.[58]
Chromosomal chimeras
These are chimeras in which the layers differ in their chromosome constitution. Occasionally, chimeras arise from loss or gain of individual chromosomes or chromosome fragments owing to misdivision.[59] More commonly cytochimeras have simple multiple of the normal chromosome complement in the changed layer. There are various effects on cell size and growth characteristics.
Nuclear gene-differential chimeras
These chimeras arise by spontaneous or induced mutation of a nuclear gene to a dominant or recessive allele. As a rule, one character is affected at a time in the leaf, flower, fruit, or other parts.
Plastid gene-differential chimeras
These chimeras arise by spontaneous or induced mutation of a plastid gene, followed by the sorting-out of two kinds of plastid during vegetative growth. Alternatively, after selfing or nucleic acid thermodynamics, plastids may sort-out from a mixed egg or mixed zygote respectively. This type of chimera is recognized at the time of origin by the sorting-out pattern in the leaves. After sorting-out is complete, periclinal chimeras are distinguished from similar looking nuclear gene-differential chimeras by their non-mendelian inheritance. The majority of variegated-leaf chimeras are of this kind.
All plastid gene- and some nuclear gene-differential chimeras affect the color of the plasmids within the leaves, and these are grouped together as chlorophyll chimeras, or preferably as variegated leaf chimeras. For most variegation, the mutation involved is the loss of the chloroplasts in the mutated tissue, so that part of the plant tissue has no green pigment and no photosynthetic ability. This mutated tissue is unable to survive on its own, but it is kept alive by its partnership with normal photosynthetic tissue. Sometimes chimeras are also found with layers differing in respect of both their nuclear and their plastid genes.
Origins
There are multiple reasons to explain the occurrence of plant chimera during plant recovery stage:
(1) The process of shoot organogenesis starts form the multicellular origin.[60]
(2) The endogenous tolerance leads to the ineffectiveness of the weak selective agents.
(3) A self-protection mechanism (cross protection). Transformed cells serve as guards to protect the untransformed ones.[61]
(4) The observable characteristic of transgenic cells may be a transient expression of the marker gene. Or it may due to the presence of agrobacterium cells.
Detection
Untransformed cells should be easy to detect and remove to avoid chimeras. This is because it is important to maintain the stable ability of the transgenic plants across different generations. Reporter genes such as GUS and Green Fluorescent Protein[62] (GFP) are utilized in combination with plant selective markers (herbicide, antibody etc.) However, GUS expression depends on the plant development stage and GFP may be influenced by the green tissue autofluorescence. Quantitative PCR could be an alternative method for chimera detection.[63]
Viruses
.jpg.webp)
In 2012, the first example of an RNA-DNA hybrid virus was unexpectedly discovered during a metagenomic study of the acidic extreme environment of Boiling Springs Lake that is in Lassen Volcanic National Park, California.[64][65] The virus was named BSL-RDHV (Boiling Spring Lake RNA DNA Hybrid Virus).[66]
Its genome is related to a DNA circovirus, which usually infect birds and pigs, and a RNA tombusvirus, which infect plants. The study surprised scientists, because DNA and RNA viruses vary and the way the chimera came together was not understood.[64][67] Other viral chimeras have also been found, and the group is known as the CHIV viruses ("chimeric viruses").[68]
Ethics and legislation
Ethics
The US and Western Europe have strict codes of ethics and regulations in place that expressly forbid certain subsets of experimentation using human cells, though there is a vast difference in the regulatory framework.[69] Through the creation of human chimeras comes the question: where does society now draw the line of humanity? This question poses serious legal and moral issues, along with creating controversy. Chimpanzees, for example, are not offered any legal standing, and are put down if they pose a threat to humans. If a chimpanzee is genetically altered to be more similar to a human, it may blur the ethical line between animal and human. Legal debate would be the next step in the process to determine whether certain chimeras should be granted legal rights.[70] Along with issues regarding the rights of chimeras, individuals have expressed concern about whether or not creating human chimeras diminishes the dignity of being human.[71]
The Human Chimera Prohibition Act
On 11 July 2005, a bill known as The Human Chimera Prohibition Act, was introduced into the United States Congress by Senator Samuel Brownback; however, it died in Congress sometime in the next year. The bill was introduced based on the findings that science has progressed to the point where the human and nonhuman species can be merged to create new forms of life. Because of this, serious ethical issues arise as this blurs the line between humans and other animals, and according to the bill with this blurring of the lines comes a show of disrespect for human dignity. The final claim brought up in The Human Chimera Prohibition Act was that there is an increasing amount of zoonotic diseases. With that being said, the creation of human-animal chimeras can allow these diseases to reach humans.[71]
On 22 August 2016, another bill, The Human-Animal Chimera Prohibition Act of 2016, was introduced to the United States House of Representatives. It identifies a chimera as:
- a human embryo into which a nonhuman cell or cells (or the component parts thereof) have been introduced to render the embryo's membership in the species Homo sapiens uncertain;
- a chimera human/animal embryo produced by fertilizing a human egg with nonhuman sperm;
- chimera human/animal embryo produced by fertilizing a nonhuman egg with human sperm;
- an embryo produced by introducing a nonhuman nucleus into a human egg;
- an embryo produced by introducing a human nucleus into a nonhuman egg;
- an embryo containing at least haploid sets of chromosomes from both a human and a nonhuman life form;
- a nonhuman life form engineered such that human gametes develop within the body of a nonhuman life form; or
- a nonhuman life form engineered such that it contains a human brain or a brain derived wholly or predominantly from human neural tissues.
The bill prohibits the attempts to create a human-animal chimera, the transfer or attempt to transfer a human embryo into a nonhuman womb, the transfer or attempt to transfer a nonhuman embryo into a human womb, and the transport or receive of any purpose of an animal chimera. Penalties for violations of this bill include fines and/or imprisonment of up to 10 years. The bill was referred to the Subcommittee on Crime, Terrorism, Homeland Security, and Investigations on October 11, 2016, but died there.[72]
See also
References
- Norton, Aaron; Ozzie Zehner (2008). "Which Half Is Mommy?: Tetragametic Chimerism and Trans-Subjectivity". Women's Studies Quarterly. Fall/Winter (3–4): 106–127. doi:10.1353/wsq.0.0115. S2CID 55282978.
- Friedman, Lauren. "The Stranger-Than-Fiction Story Of A Woman Who Was Her Own Twin". Retrieved 4 August 2014.
- Strain, Lisa; John C.S. Dean; Mark P. R. Hamilton; David T. Bonthron (1998). "A True Hermaphrodite Chimera Resulting from Embryo Amalgamation after in Vitro Fertilization". The New England Journal of Medicine. 338 (3): 166–169. doi:10.1056/NEJM199801153380305. PMID 9428825.
- Schoenle, E.; Schmid, W.; Schinzel, A.; Mahler, M.; Ritter, M.; Schenker, T.; Metaxas, M.; Froesch, P.; Froesch, E. R. (1983-07-01). "46,XX/46,XY chimerism in a phenotypically normal man". Human Genetics. 64 (1): 86–89. doi:10.1007/BF00289485. ISSN 1432-1203. PMID 6575956. S2CID 25946104.
- Binkhorst, Mathijs; de Leeuw, Nicole; Otten, Barto J. (January 2009). "A healthy, female chimera with 46,XX/46,XY karyotype". Journal of Pediatric Endocrinology & Metabolism. 22 (1): 97–102. doi:10.1515/jpem.2009.22.1.97. ISSN 0334-018X. PMID 19344081. S2CID 6074854.
- Gencík, A.; Genciková, A.; Hrubisko, M.; Mergancová, O. (1980). "Chimerism 46,XX/46,XY in a phenotypic female". Human Genetics. 55 (3): 407–408. doi:10.1007/bf00290226. ISSN 0340-6717. PMID 7203474. S2CID 9117759.
- Farag, T I; Al-Awadi, S A; Tippett, P; el-Sayed, M; Sundareshan, T S; Al-Othman, S A; el-Badramany, M H (December 1987). "Unilateral true hermaphrodite with 46,XX/46,XY dispermic chimerism". Journal of Medical Genetics. 24 (12): 784–786. doi:10.1136/jmg.24.12.784. ISSN 0022-2593. PMC 1050410. PMID 3430558.
- Shah, V. C.; Krishna Murthy, D. S.; Roy, S.; Contractor, P. M.; Shah, A. V. (November 1982). "True hermaphrodite: 46, XX/46, XY, clinical cytogenetic and histopathological studies". Indian Journal of Pediatrics. 49 (401): 885–890. doi:10.1007/bf02976984. ISSN 0019-5456. PMID 7182365. S2CID 41204037.
- Hadjiathanasiou, C. G.; Brauner, R.; Lortat-Jacob, S.; Nivot, S.; Jaubert, F.; Fellous, M.; Nihoul-Fékété, C.; Rappaport, R. (November 1994). "True hermaphroditism: genetic variants and clinical management". The Journal of Pediatrics. 125 (5 Pt 1): 738–744. doi:10.1016/s0022-3476(94)70067-2. ISSN 0022-3476. PMID 7965425.
- Boklage, C.E. How New Humans Are Made. Hackensack, NJ; London: World Scientific Publishing Co. Pte. Ltd; 2010
- Ross, C. N.; J. A. French; G. Orti (2007). "Germ-line chimerism and paternal care in marmosets (Callithrix kuhlii)". Proceedings of the National Academy of Sciences. 104 (15): 6278–6282. Bibcode:2007PNAS..104.6278R. doi:10.1073/pnas.0607426104. ISSN 0027-8424. PMC 1851065. PMID 17389380.
- Masahito Tachibana, Michelle Sparman and Shoukhrat Mitalipov (January 2012). "Generation of Chimeric Rhesus Monkeys". Cell. 148 (1–2): 285–95. doi:10.1016/j.cell.2011.12.007. PMC 3264685. PMID 22225614.
- Gengozian, N.; Batson, JS; Eide, P. (1964). "Hematologic and Cytogenetic Evidence for Hematopoietic Chimerism in the Marmoset, Tamarinus Nigricollis". Cytogenetics. 10 (6): 384–393. doi:10.1159/000129828. PMID 14267132.
- Starr, Barry (November 30, 2004). "Understanding Genetics: Human Health and the Genome". Ask a Geneticist. Stanford University School of Medicine. Archived from the original on 2011-07-24.
- "The Twin Inside Me: Extraordinary People". Channel 5 TV, UK. 9 March 2006. Archived from the original on May 26, 2006.
- Bellefon, L.; Heiman, P.; Kanaan, S.; Azzouz, D.; Rak, J.; Martin, M.; Roudier, J.; Roufosse, F.; Lambert, C. (2010). "Cells from a vanished twin as a source of microchimerism 40 years later". Chimerism. 1 (2): 56–60. doi:10.4161/chim.1.2.14294. PMC 3023624. PMID 21327048.
- Ceratiidae
- Zimmer, Carl (2007-03-27). "In the Marmoset Family, Things Really Do Appear to Be All Relative". The New York Times. Retrieved 2010-04-01.
- Hooper, Rowan (26 March 2007). "Marmosets may carry their sibling's sex cells". New Scientist.
- Rinkevich, B. (June 2001). "Human natural chimerism: an acquired character or a vestige of evolution?". Human Immunology. 62 (6): 651–657. doi:10.1016/s0198-8859(01)00249-x. ISSN 0198-8859. PMID 11390041.
- "Developmental Biology Cinema, Le Douarin". sdbonline.org. Retrieved 2020-04-10.
- Ballantyne, KN; Kayser, M; Grootegoed, JA (2011). "Sex and gender issues in competitive sports: investigation of a historical case leads to a new viewpoint". British Journal of Sports Medicine. 46 (8): 614–7. doi:10.1136/bjsm.2010.082552. PMC 3375582. PMID 21540190.
- Bowley, C. C.; Ann M. Hutchison; Joan S. Thompson; Ruth Sanger (July 11, 1953). "A human blood-group chimera". British Medical Journal. 2 (4827): 81. doi:10.1136/bmj.2.4827.81. PMC 2028470. PMID 13051584.
- Van Dijk, B. A.; Boomsma, D. I.; De Man, A. J. (1996). "Blood group chimerism in human multiple births is not rare". American Journal of Medical Genetics. 61 (3): 264–8. CiteSeerX 10.1.1.149.9001. doi:10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. PMID 8741872.
- "She's Her Own Twin". ABC News. August 15, 2006. Archived from the original on October 28, 2013. Retrieved September 17, 2013.
- Yu, Neng; Kruskall, Margot S.; Yunis, Juan J.; Knoll, Joan H.M.; Uhl, Lynne; Alosco, Sharon; Ohashi, Marina; Clavijo, Olga; Husain, Zaheed; Yunis, Emilio J.; Yunis, Jorge J. (2002-05-16). "Disputed Maternity Leading to Identification of Tetragametic Chimerism". New England Journal of Medicine. 346 (20): 1545–1552. doi:10.1056/NEJMoa013452. ISSN 0028-4793. PMID 12015394.
- Gallagher, James (2017-01-26). "Human-pig 'chimera embryos' detailed". BBC News. Retrieved 2017-06-03.
- "Human-Pig Hybrid Created in the Lab—Here Are the Facts". 2017-01-26. Retrieved 2017-06-03.
- "Scientists create human/pig hybrid". The Independent. January 26, 2017. Retrieved 2017-06-03.
- Bayraktar, Zeki (2018). "Potential autofertility in true hermaphrodites". The Journal of Maternal-Fetal & Neonatal Medicine. 31 (4): 542–547. doi:10.1080/14767058.2017.1291619. PMID 28282768. S2CID 22100505.
- Murphy, Heather (2019-12-09). "Man who had transplant finds out months later his DNA has changed to that of donor 5,000 miles away". The Guardian. Retrieved December 12, 2019.
- Murphy, Erin E. (2015-10-07). "DNA at the Fringes: Twins, Chimerism, and Synthetic DNA". The Daily Beast.
- Schlueter, Roger (2018-02-01). "Bone marrow transplant could give you new DNA". Herald & Review. Retrieved 2020-02-08.
- "Chimerism: Definition, Symptoms, Testing, Diagnosis, and More". Healthline. Retrieved 2020-03-15.
- "National Society of Genetic Counselors : Blogs : Chimerism Explained: How One Person Can Unknowingly Have Two Sets of DNA". nsgc.org. Retrieved 2020-03-15.
- "It's a Geep". Time. 27 February 1984. Retrieved 4 January 2012.
- Mott, Maryann (January 25, 2005). "Animal-Human Hybrids Spark Controversy". National Geographic News.
- "Iranian scientist creates sheep with half-human organs". Press TV. 27 Mar 2007. Archived from the original on November 14, 2007.
- "Chimerism Explained: How One Person Can Unknowingly Have Two Sets of DNA". National Society of Genetic Counselors.
- Blanquer, Andrea; Uriz, Maria-J. (2011-04-15). "'Living Together Apart': The Hidden Genetic Diversity of Sponge Populations". Molecular Biology and Evolution. 28 (9): 2435–2438. doi:10.1093/molbev/msr096. ISSN 1537-1719. PMID 21498599.
- Mintz, B.; Silvers, W. K. (1967). "'Intrinsic' Immunological Tolerance in Allophenic Mice". Science. 158 (3807): 1484–6. Bibcode:1967Sci...158.1484M. doi:10.1126/science.158.3807.1484. PMID 6058691. S2CID 23824274.
- Robertson, EJ (1986). "Pluripotential stem cell lines as a route into the mouse germ line". Trends Genet. 2: 9–13. doi:10.1016/0168-9525(86)90161-7.
- Doetschman, T.; Maeda, N.; Smithies, O. (1988). "Targeted mutation of the Hp gene in mouse embryonic stem cells". Proc. Natl. Acad. Sci. 85 (22): 8583–8587. Bibcode:1988PNAS...85.8583D. doi:10.1073/pnas.85.22.8583. PMC 282503. PMID 3186749.
- Ralston, A; Rossant, J (2005). "Genetic regulation of stem cell origins in the mouse embryo". Clin Genet. 68 (2): 106–112. doi:10.1111/j.1399-0004.2005.00478.x. PMID 15996204.
- Tam, P.L.; Rossant, J. (2003). "Mouse embryonic chimeras: tools for studying mammalian development". Development. 130 (25): 6155–6163. doi:10.1242/dev.00893. PMID 14623817.
- Rossant, J. (1976). "Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs". J. Embryol. Exp. Morphol. 36 (2): 283–290. PMID 1033982.
- Pappaioannou, V.; Johnson, R. (1993). Joyner, A. (ed.). "Production of chimeras and genetically defined offspring from targeted ES cells". Gene Targeting: A Practical Approach. IRL Press at Oxford University Press: 107–146.
- Kubiak, J; Tarkowski, A. (1985). "Electrofusion of mouse blastomeres. Exp". Cell Res. 157 (2): 561–566. doi:10.1016/0014-4827(85)90143-0. PMID 3884349.
- Nagy, A.; Rossant, J. (1999). Joyner, A. (ed.). "Production of Es-cell aggregation chimeras". Gene Targeting: A Practical Approach. IRL Press at Oxford University Press: 107–205.
- Jasin, M; Moynahan, ME; Richardson, C (1996). "Targeted transgenesis". PNAS. 93 (17): 8804–8808. Bibcode:1996PNAS...93.8804J. doi:10.1073/pnas.93.17.8804. PMC 38547. PMID 8799106.
- Ledermann, B (2000). "Embryonic Stem Cell and Gene Targeting". Experimental Physiology. 85 (6): 603–613. doi:10.1017/S0958067000021059. PMID 11187956.
- Chimera Mouse production by blastocyst injection, Wellcome trust Sanger Institute, http://www.eucomm.org/docs/protocols/mouse_protocol_1_Sanger.pdf
- Tanaka, M; Hadjantonakis, AK; Nagy, A (2001). Aggregation chimeras. Combining ES cells, diploid and tetraploid embryos. Methods in Molecular Biology. 158. pp. 135–54. doi:10.1385/1-59259-220-1:135. ISBN 978-1-59259-220-3. PMID 11236654.
- Kirk, John Thomas Osmond; Tilney-Bassett, Richard A. E. (1978). The plastids, their chemistry, structure, growth, and inheritance (Rev. 2d ed.). Elsevier/North Holland Biomedical Press. ISBN 9780444800220. Retrieved 9 February 2020.
- van Harten, A. M. (1978). "Mutation Breeding Techniques and Behaviour of Irradiated Shoot Apices of Potato". Agricultural Research Reports. Wageningen, Netherlands: Centre for Agricultural Publishing and Documentation (PUDOC) (873). ISBN 978-90-220-0667-2. Retrieved 9 February 2020.
- Norris, R.; Smith, R.H. & Vaughn, K.C. (1983). "Plant chimeras used to establish de novo origin of shoots". Science. 220 (4592): 75–76. Bibcode:1983Sci...220...75N. doi:10.1126/science.220.4592.75. PMID 17736164. S2CID 38143321.
- Tilney-Bassett, Richard A. E. (1991). Plant Chimeras. Cambridge University Press. ISBN 978-0-521-42787-6. Retrieved 9 February 2020.
- "Growing Fruit: Grafting Fruit Trees in the Home Orchard [fact sheet] | UNH Extension". extension.unh.edu. Retrieved 2020-02-23.
- Thompson, J.D.; Herre, E.A.; Hamrick, J.L. & Stone, J.L. (1991). "Genetic Mosaics in strangler Fig Trees: Implication for Tropical Conservation". Science. 254 (5035): 1214–1216. Bibcode:1991Sci...254.1214T. doi:10.1126/science.254.5035.1214. PMID 17776412. S2CID 40335585.
- Zhu, X.; Zhao, M.; Ma, S.; Ge, Y.; Zhang, M. & Chen, L. (2007). "Induction and origin of adventitious shoots from chimeras of Brassica juncea and Brassica oleracea". Plant Cell Rep. 26 (10): 1727–1732. doi:10.1007/s00299-007-0398-4. PMID 17622536. S2CID 23069396.
- Park SH, Rose SC, Zapata C, Srivatanakul M (1998). "Cross-protection and selectable marker genes in plant transformation". In Vitro Cellular & Developmental Biology - Plant. 34 (2): 117–121. doi:10.1007/BF02822775. S2CID 30883689.
- Rakosy-Tican, E.; Aurori, C.M.; Dijkstra, C.; Thieme, R.; Aurori, A. & Davey, M.R. (2007). "The usefulness of the gfp reporter gene for monitoring Agrobacterium-mediated transformation of potato dihaploid and tetraploid genotypes". Plant Cell Rep. 26 (5): 661–671. doi:10.1007/s00299-006-0273-8. PMID 17165042. S2CID 30548375.
- Faize, M.; Faize, L.; Burgos, L. (2010). "Using quantitative real-time PCR to detect chimeras in transgenic tobacco and apricot and to monitor their dissociation". BMC Biotechnology. 10 (1): 53. doi:10.1186/1472-6750-10-53. PMC 2912785. PMID 20637070.
- Diemer, Geoffrey S., Kenneth M. (11 June 2013). "A novel virus genome discovered in an extreme environment suggests recombination between unrelated groups of RNA and DNA viruses". Biology Direct. Retrieved 29 March 2020.
- Thompson, Helen (20 April 2012). "Hot spring yields hybrid genome: Researchers discover natural chimaeric DNA-RNA virus". Nature. Retrieved 27 March 2020.
- Devor, Caitlin (12 July 2012)."Scientists discover hybrid virus". Journal of Young Investigators". Retrieved 31 March 2020.
- BioMed Central Limited (18 April 2012). "Could a newly discovered viral genome change what we thought we knew about virus evolution?". ScienceDaily. Retrieved March 31, 2020.
- Koonina, Eugene V., Doljab, Valerian V., and Krupovic, Mart. (May 2015)."Origins and evolution of viruses of eukaryotes: The ultimate modularity". Virology. p. 26. Retrieved March 31, 2020.
- Futehally, Ilmas, Beyond Biology, Strategic Foresight Group
- Bruch, Quinton (2014-02-20). "Defining Humanity: The Ethics of Chimeric Animals and Organ Growing". The Triple Helix Online. Retrieved 21 May 2015.
- Brownback, Samuel (2005-03-17). "S.659 – Human Chimera Prohibition Act of 2005 (Introduced in Senate - IS)". The Library of Congress THOMAS. Retrieved 20 May 2015.
- Smith, Christopher H. (2016-10-11). "Text - H.R.6131 - 114th Congress (2015-2016): Human-Animal Chimera Prohibition Act of 2016". congress.gov. Retrieved 2019-11-14.
Further reading
- Yu N, Kruskall MS, Yunis JJ, Knoll JH, Uhl L, Alosco S, Ohashi M, Clavijo O, Husain Z, Yunis EJ, Yunis JJ, Yunis EJ (2002). "Disputed maternity leading to identification of tetragametic chimerism". N Engl J Med. 346 (20): 1545–52. doi:10.1056/NEJMoa013452. PMID 12015394.
- Appel, Jacob M. "The Monster's Law", Genewatch, Volume 19, Number 2, March–April 2007.
- Nelson, J. Lee (Scientific American, February 2008). Your Cells Are My Cells
- Weiss, Rick (August 14, 2003). Cloning yields human-rabbit hybrid embryo. The Washington Post.
- Weiss, Rick (February 13, 2005). U.S. Denies Patent for a too-human hybrid. The Washington Post.
- L. M. Repas-Humpe; A. Humpe; R. Lynen; B. Glock; E. M. Dauber; G. Simson; W. R. Mayr; M. Köhler; S. Eber (1999). "A Dispermic Chimerism in a 2-year-old Caucasian Boy". Annals of Hematology. 78 (9): 431–434. doi:10.1007/s002770050543. PMID 10525832. S2CID 24655050.
- Strain, Lisa; Dean, John C.S.; Hamilton, Mark P.R.; Bonthron, David T. (1998). "A True Hermaphrodite Chimera Resulting from Embryo Amalgamation after in Vitro Fertilization". New England Journal of Medicine. 338 (3): 166–9. doi:10.1056/NEJM199801153380305. PMID 9428825.
- Jones, David Albert; MacKellar, Calum, eds. (2012). Chimera's Children: Ethical, Philosophical and Religious Perspectives on Human-Nonhuman Experimentation. London: Continuum Books. ISBN 9781441195807.
External links
| Wikimedia Commons has media related to Chimera (genetics). |
- "Chimerism Explained"
- Chimerism and cellular mosaicism, Genetic Home Reference, U.S. National Library of Medicine, National Institute of Health.
- Chimera: Apical Origin, Ontogeny and Consideration in Propagation
- Plant Chimeras in Tissue Culture
- Ainsworth, Claire (November 15, 2003). "The Stranger Within". New Scientist (subscription required). (Reprinted here )