Copper toxicity
Copper toxicity is a type of metal poisoning caused by an excess of copper in the body. Copperiedus can occur from eating acidic foods cooked in uncoated copper cookware, an IUD, or from exposure to excess copper in drinking water and other environmental sources .
| Copper toxicity | |
|---|---|
| Other names | Copperiedus |
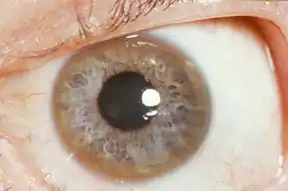 | |
| A Kayser-Fleischer ring, copper deposits found in the cornea, is an indication the body is not metabolizing copper properly. | |
| Specialty | Toxicology |
Signs and symptoms
Acute symptoms of copper poisoning by ingestion include vomiting, hematemesis (vomiting of blood), hypotension (low blood pressure), melena (black "tarry" feces), coma, jaundice (yellowish pigmentation of the skin), and gastrointestinal distress.[1] Individuals with glucose-6-phosphate deficiency may be at increased risk of hematologic effects of copper.[1] Hemolytic anemia resulting from the treatment of burns with copper compounds is infrequent.[1]
Chronic (long-term) copper exposure can damage the liver and kidneys.[2] Mammals have efficient mechanisms to regulate copper stores such that they are generally protected from excess dietary copper levels.[2][3]
Those same protection mechanisms can cause milder symptoms, which are often misdiagnosed as psychiatric disorders. There is a lot of research on the function of the Cu/Zn ratio in neurological, endocrinological, and psychological conditions.[4][5][6] Many of the substances that protect us from excess copper perform important functions in our neurological and endocrine systems, leading to diagnostic difficulties. When they are used to bind copper in the plasma, to prevent it from being absorbed in the tissues, their own function may go unfulfilled. Such symptoms often include mood swings, irritability, depression, fatigue, excitation, difficulty focusing, and feeling out of control. To further complicate diagnosis, some symptoms of excess copper are similar to those of a copper deficit.
The U.S. Environmental Protection Agency's Maximum Contaminant Level (MCL) in drinking water is 1.3 milligrams per liter.[1][7] The MCL for copper is based on the expectation that a lifetime of consuming copper in water at this level is without adverse effect (gastrointestinal). The US EPA lists copper as a micronutrient and a toxin.[8] Toxicity in mammals includes a wide range of animals and effects such as liver cirrhosis, necrosis in kidneys and the brain, gastrointestinal distress, lesions, low blood pressure, and fetal mortality.[9][10][11] The Occupational Safety and Health Administration (OSHA) has set a limit of 0.1 mg/m3 for copper fumes (vapor generated from heating copper) and 1 mg/m3 for copper dusts (fine metallic copper particles) and mists (aerosol of soluble copper) in workroom air during an eight-hour work shift, 40-hour work week.[12] Toxicity to other species of plants and animals is noted to varying levels.[8]
Toxicity
Copper in the blood and blood stream exists in two forms: bound to ceruloplasmin (85–95%), and the rest "free", loosely bound to albumin and small molecules. Nutritionally, there is a distinct difference between organic and inorganic copper, according to whether the copper ion is bound to an organic ligand.[13][14]
EPA cancer data
The EPA lists no evidence for human cancer incidence connected with copper, and lists animal evidence linking copper to cancer as "inadequate". Two studies in mice have shown no increased incidence of cancer. One of these used regular injections of copper compounds, including cupric oxide. One study of two strains of mice fed copper compounds found a varying increased incidence of reticulum cell sarcoma in males of one strain, but not the other (there was a slightly increased incidence in females of both strains). These results have not been repeated.[15]
Cause
Cookware
Cookware in which copper is the main structural element (as opposed to copper clad, copper sandwiched or copper colored) is sometimes manufactured without a lining when intended to be used for any of a number of specific culinary tasks, such as preparing preserves or meringues. Otherwise, copper cookware is lined with a non-reactive metal to prevent contact between acidic foods and the structural copper element of the cookware.
Excepting for acute or chronic conditions, exposure to copper in cooking is generally considered harmless.[16] According to Paracelsus, dosage makes the poison; as this pertains to copper "a defense mechanism has apparently evolved as a consequence of which toxicity in man is very unusual."[17]
Acute exposure and attendant copper toxicity is possible when cooking or storing highly acidic foods in unlined copper vessels for extended periods, or by exposing foodstuffs to reactive copper salts (copper corrosion, or verdigris). Continuous, small ("chronic") exposures of acidic foods to copper may also result in toxicity in cases where either surface area interaction potentials are significant, pH is exceptionally low and concentrated (in the case of cooking with, for example, vinegar or wine), or both, and insufficient time elapses between exposures for normal homeostatic elimination of excess copper.
Exceptions to the above may be observed in the case of jam, jelly and preserve -making, wherein unlined copper vessels are used to cook (not to store) acidic preparations, in this case of fruit. Methods of jamming and preserving specify sugar as chemically necessary to the preserving (antibacterial) action, which has the additional effect of mediating (buffering) the interaction of fruit acid with copper,[18] permitting the use of the metal for its efficient thermal transfer properties.[19]
Non-sparking tools
OSHA has set safety standards for grinding and sharpening copper and copper alloy tools, which are often used in nonsparking applications. These standards are recorded in the Code of Federal Regulations 29 CFR 1910.134 and 1910.1000.[20]
Note: The most important nonsparking copper alloy is beryllium copper, and can lead to beryllium poisoning.
Drinking water
With an LD50 of 30 mg/kg in rats, "gram quantities" of copper sulfate are potentially lethal in humans.[21] The suggested safe level of copper in drinking water for humans varies depending on the source, but tends to be pegged at 1.3 mg/l.[22]
Birth control
There are conditions in which an individual's copper metabolism is compromised to such an extent that birth control may cause an issue with copper accumulation. They include toxicity or just increased copper from other sources, as well as the increased copper level of the individual's mother via the placenta before birth.[23]
Pathophysiology
A significant portion of the toxicity of copper comes from its ability to accept and donate single electrons as it changes oxidation state. This catalyzes the production of very reactive radical ions, such as hydroxyl radical in a manner similar to Fenton chemistry.[24] This catalytic activity of copper is used by the enzymes with which it is associated, thus is only toxic when unsequestered and unmediated. This increase in unmediated reactive radicals is generally termed oxidative stress, and is an active area of research in a variety of diseases where copper may play an important but more subtle role than in acute toxicity.
Some of the effects of aging may be associated with excess copper.[25]
Indian childhood cirrhosis
One manifestation of copper toxicity, cirrhosis of the liver in children (Indian childhood cirrhosis), has been linked to boiling milk in copper cookware. The Merck Manual states that recent studies suggest that a genetic defect is associated with this particular cirrhosis.[26]
Wilson's disease
An inherited condition called Wilson's disease causes the body to retain copper, since it is not excreted by the liver into the bile. This disease, if untreated, can lead to brain and liver damage, and bis-choline tetrathiomolybdate is under investigation as a therapy against Wilson's disease.
Alzheimer's disease
Elevated free copper levels exist in Alzheimer's disease,[27] which has been hypothesized to be linked to inorganic copper consumption.[28] Copper and zinc are known to bind to amyloid beta proteins in Alzheimer's disease.[29] This bound form is thought to mediate the production of reactive oxygen species in the brain.[30]
Diagnosis
ICD-9-CM
ICD-9-CM code 985.8 Toxic effect of other specified metals includes acute & chronic copper poisoning (or other toxic effect) whether intentional, accidental, industrial etc.
- In addition, it includes poisoning and toxic effects of other metals including tin, selenium, nickel, iron, heavy metals, thallium, silver, lithium, cobalt, aluminum and bismuth. Some poisonings, e.g. zinc phosphide, would/could also be included as well as under 989.4 Poisoning due to other pesticides, etc.
- Excluded are toxic effects of mercury, arsenic, manganese, beryllium, antimony, cadmium, and chromium.
ICD-10-CM
| Code | Term |
|---|---|
| T56.4X1D | Toxic effect of copper and its compounds, accidental (unintentional), subsequent encounter |
| T56.4X1S | Toxic effect of copper and its compounds, accidental (unintentional), sequela |
| T56.4X2D | Toxic effect of copper and its compounds, intentional self-harm, subsequent encounter |
| T56.4X2S | Toxic effect of copper and its compounds, intentional self-harm, sequela |
| T56.4X3D | Toxic effect of copper and its compounds, assault, subsequent encounter |
| T56.4X3S | Toxic effect of copper and its compounds, assault, sequela |
| T56.4X4D | Toxic effect of copper and its compounds, undetermined, subsequent encounter |
| T56.4X4S | Toxic effect of copper and its compounds, undetermined, sequela |
SNOMED
| Concept ID | Term |
|---|---|
| 46655005 | Copper |
| 43098002 | Copper fever |
| 49443005 | Phytogenous chronic copper poisoning |
| 50288007 | Chronic copper poisoning |
| 73475009 | Hepatogenous chronic copper poisoning |
| 875001 | Chalcosis of eye |
| 90632001 | Acute copper poisoning |
Treatment
In cases of suspected copper poisoning, penicillamine is the drug of choice, and dimercaprol, a heavy metal chelating agent, is often administered. Vinegar is not recommended to be given, as it assists in solubilizing insoluble copper salts. The inflammatory symptoms are to be treated on general principles, as are the nervous ones.
There is some evidence that alpha-lipoic acid (ALA) may work as a milder chelator of tissue-bound copper.[31] Alpha lipoic acid is also being researched for chelating other heavy metals, such as mercury.[32]
Aquatic life
Too much copper in water may damage marine and freshwater organisms such as fish and molluscs.[33] Fish species vary in their sensitivity to copper, with the LD50 for 96-h exposure to copper sulphate reported to be in the order of 58 mg per litre for Tilapia (Oreochromis niloticus) and 70 mg per litre for catfish (Clarias gariepinus) [34] The chronic effect of sublethal concentrations of copper on fish and other creatures is damage to gills, liver, kidneys and the nervous system. It also interferes with the sense of smell in fish, thus preventing them from choosing good mates or finding their way to mating areas.[35]
Copper-based paint is a common marine antifouling agent.[36] In the United States, copper-based paint replaced tributyltin, which was banned due to its toxicity, as a way for boats to control organic growth on their hulls. In 2011, Washington state became the first U.S. state to ban the use of copper-based paint for boating, although it only applied to recreational boats.[37] California has also pursued initiatives to reduce the effect of copper leaching, with the U.S. EPA pursuing research.[38]
Copper is an essential elemental for metabolic processes in marine algae. It is required for electron transport in photosynthesis and by various enzyme systems. Too much copper can also affect phytoplankton or marine algae in both marine and freshwater ecosystems. It has been show to inhibit photosynthesis, disrupt electron transport in photosystem 2, reduce pigment concentrations, restrict growth, reduce reproduction, etc. [39] The toxicity of Copper is widely recognized and is used to help prevent algal blooms. The effect of Copper is solely dependent on the free Copper the water is receiving. It's determined by the relative solubility and the concentration of the Copper binding ligands. Based on that, they can look at both natural and anthropogenic situations. They have done studies to show that Copper concentrations are toxic when marine phytoplankton are confined to areas that are heavily impacted by anthropogenic emissions. [40] Some of the studies have used a marine amphipod to show how Copper affects it. This particular study said that the juveniles were 4.5 more times sensitive to the toxins than the adults.[41] Another study used 7 different algal species. They found that one species was more sensitive than the others, which was Synechococcus, and that another species was more sensitive in seawater, which wasThalassiosira weissflogii. [42]
One study used cyanobacteria, diatoms, coccolithophores, and dinoflagellates. This study showed that cyanobacteria was the most sensitive, diatoms were the least sensitive, and the coccolithophores and dinoflagellates were intermediate. They used copper ion in a buffer system and controlled it at different levels. They found that cyanobacteria reproduction rates were reduced while other algae had maximum reproduction rates. They found that Copper may influence seasonal successions of species. [43]
Bacteria
Copper and copper alloys such as brass have been found to be toxic to bacteria via the oligodynamic effect. The exact mechanism of action is unknown, but common to other heavy metals. Viruses are less susceptible to this effect than bacteria. Associated applications include the use of brass doorknobs in hospitals, which have been found to self-disinfect after eight hours, and mineral sanitizers, in which copper can act as an algicide. Overuse of copper sulfate as an algicide has been speculated to have caused a copper poisoning epidemic on Great Palm Island in 1979.[44]
References
- Casarett, L.; Casarett, L.J.; Amdur, M.O.; Doull, J. (1996). Casarett & Doull's Toxicology, The Basic Science of Poisons (5th ed.). McGraw-Hill. p. 715. ISBN 0071054766.
- "Copper: Health Information Summary" (PDF). Environmental Fact Sheet. New Hampshire Department of Environmental Services. 2005. ARD-EHP-9.
- Lutsenko, Svetlana; Barnes, Natalie L.; Bartee, Mee Y.; Dmitriev, Oleg Y. (2007). "Function and Regulation of Human Copper-Transporting ATPases". Physiological Reviews. 87 (3): 1011–46. doi:10.1152/physrev.00004.2006. PMID 17615395.
- Desai, Vishal; Kaler, Stephen G. (2008). "Role of copper in human neurological disorders". The American Journal of Clinical Nutrition. 88 (3): 855S–8S. doi:10.1093/ajcn/88.3.855S. PMID 18779308. Retrieved 20 December 2015.
- Kaplan, Bonnie J.; Crawford, Susan G.; Gardner, Beryl; Farrelly, Geraldine (2002). "Treatment of Mood Lability and Explosive Rage with Minerals and Vitamins: Two Case Studies in Children". Journal of Child and Adolescent Psychopharmacology. 12 (3): 205–219. doi:10.1089/104454602760386897. PMID 12427294.
- Faber, Scott; Zinn, Gregory M.; Kern Ii, John C.; Skip Kingston, H. M. (2009). "The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders". Biomarkers. 14 (3): 171–180. doi:10.1080/13547500902783747. PMID 19280374. S2CID 205770002.
- Federal Register / Vol. 65, No. 8 / Wednesday, January 12, 2000 / Rules and Regulations. pp. 1976.
- US EPA Region 5 (2011-12-28). "Ecological Toxicity Information". US EPA. Retrieved 17 June 2015.
- "Toxicological Profile for Copper". Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services. Retrieved 17 June 2015.
- Kabata-Pendias, Alina (2010). Trace Elements in Soils and Plants, Fourth Edition (4th ed.). Taylor & Francis. ISBN 9781420093681. Archived from the original on 16 July 2015. Retrieved 17 June 2015.
- Ware, George W. (1983). Pesticides: Theory and application. New York: W.H. Freeman. OCLC 669712126.
- Occupational Safety and Health Administration, U.S. Department of Labor, Copper, Available Online at: https://www.osha.gov/SLTC/metalsheavy/copper.html
- Batley, G.E.; Florence, T.M. (1976). "Determination of the chemical forms of dissolved cadmium, lead and copper in seawater". Marine Chemistry. 4 (4): 347–363. doi:10.1016/0304-4203(76)90020-7.
- Van Den Berg, C.M. (1984). "Organic and inorganic speciation of copper in the Irish Sea". Marine Chemistry. 14 (3): 201–212. doi:10.1016/0304-4203(84)90042-2.
- EPA results for copper and cancer. Accessed March 11, 2011
- "The Dispatch - Google News Archive Search".
- Sternlieb, Irmin (June 1967). "Gastrointestinal Copper Absorption in Man". Gastroenterology. 52 (6): 1038–1041. doi:10.1016/S0016-5085(67)80160-4. PMID 6026483.
- "Jam Making 101: The Tools and Techniques for Success".
- Escoffier, Auguste; Gilbert, Pliléas (1961). Larousse Gastronomique. New York: Crown. p. 546.
- "Occupational Safety and Health Standards". Retrieved 2012-09-18.
- "Pesticide Information Profile for Copper Sulfate". Cornell University. Retrieved 2008-07-10.
- "The Water Supply (Water Quality) Regulations 2000".
- McArdle HJ, Andersen HS, Jones H, Gambling L (2008). "Copper and iron transport across the placenta: regulation and interactions". Journal of Neuroendocrinology. 20 (4): 427–31. doi:10.1111/j.1365-2826.2008.01658.x. PMID 18266949. S2CID 12395297.
- Held KD; et al. (May 1996). "Role of Fenton chemistry in thiol-induced toxicity and apoptosis". Radiat. Res. 145 (5): 542–53. Bibcode:1996RadR..145..542H. doi:10.2307/3579272. JSTOR 3579272. PMID 8619019.
- Brewer GJ (February 2007). "Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer's disease". Exp. Biol. Med. (Maywood). 232 (2): 323–35. PMID 17259340.
- "Copper". Merck Manuals — Online Medical Library. Merck. November 2005. Retrieved 2008-07-19.
- Brewer GJ (Apr 2010). "Copper toxicity in the general population". Clin Neurophysiol. 121 (4): 459–60. doi:10.1016/j.clinph.2009.12.015. PMID 20071223. S2CID 43106197.
- Brewer GJ (June 2009). "The risk of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer's disease". J. Am. Coll. Nutr. 28 (3): 238–42. doi:10.1080/07315724.2009.10719777. PMID 20150596. S2CID 21630019.
- Faller P (2009-12-14). "Copper and zinc binding to amyloid-beta: coordination, dynamics, aggregation, reactivity and metal-ion transfer". ChemBioChem. 10 (18): 2837–45. doi:10.1002/cbic.200900321. PMID 19877000. S2CID 35130040.
- Hureau C, Faller P (October 2009). "Abeta-mediated ROS production by Cu ions: structural insights, mechanisms and relevance to Alzheimer's disease". Biochimie. 91 (10): 1212–7. doi:10.1016/j.biochi.2009.03.013. PMID 19332103.
- Marangon, Karine; Devaraj, Sridevi; Tirosh, Oren; Packer, Lester; Jialal, Ishwarlal (November 1999). "Comparison of the effect of α-lipoic acid and α-tocopherol supplementation on measures of oxidative stress". Free Radical Biology and Medicine. 27 (9–10): 1114–1121. doi:10.1016/S0891-5849(99)00155-0. PMID 10569644.
- "Mercury toxicity and antioxidants: part I: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. (Mercury Toxicity)". Thorne Research Inc. 2002. Archived from the original on 22 December 2015. Retrieved 20 December 2015.
- Van Genderen EJ, Ryan AC, Tomasso JR, Klaine SJ (February 2005). "Evaluation of acute copper toxicity to larval fathead minnows (Pimephales promelas) in soft surface waters". Environ. Toxicol. Chem. 24 (2): 408–14. doi:10.1897/03-494.1. PMID 15720002.
- Ezeonyejiaku, CD, Obiakor, MO and Ezenwelu, CO (2011). "Toxicity of copper sulphate and behavioural locomotor response of tilapia (Oreochromis niloticus) and catfish (Clarias gariepinus) species". Online J. Anim. Feed Res. 1 (4): 130–134.CS1 maint: multiple names: authors list (link)
- C. A. Flemming; J. T. Trevors (1989). "Copper toxicity and chemistry in the environment: a review". Water, Air, & Soil Pollution. 44 (1–2): 143–158. Bibcode:1989WASP...44..143F. doi:10.1007/BF00228784. S2CID 98175996.
- Earley, Patrick J.; Swope, Brandon L.; Barbeau, Katherine; Bundy, Randelle; McDonald, Janessa A.; Rivera-Duarte, Ignacio (2014-01-01). "Life cycle contributions of copper from vessel painting and maintenance activities". Biofouling. 30 (1): 51–68. doi:10.1080/08927014.2013.841891. ISSN 0892-7014. PMC 3919178. PMID 24199998.
- "Is Copper Bottom Paint Sinking? - BoatUS Magazine". Retrieved 2016-09-22.
- "Marine Coatings: Making Sense of U.S., State, and Local Mandates of Copper-Based Antifouling Regulations". American Coatings Association. Retrieved 2016-09-22.
- https://www.researchgate.net/profile/Murray_Brown/publication/228052466_The_toxicity_of_copper_II_species_to_marine_with_particular_reference_to_macroalgae/links/5954f3990f7e9b2da1b3bce0/The-toxicity-of-copper-II-species-to-marine-with-particular-reference-to-macroalgae.pdf
- Lopez, Johann S.; Lee, Lillian; Mackey, Katherine R. M. (2019-01-24). "The Toxicity of Copper to Crocosphaera watsonii and Other Marine Phytoplankton: A Systematic Review". Frontiers in Marine Science. 5: 511. doi:10.3389/fmars.2018.00511. ISSN 2296-7745.
- Ahsanullah, M.; Florence, T. M. (1984-12-01). "Toxicity of copper to the marine amphipod Allorchestes compressa in the presence of water-and lipid-soluble ligands". Marine Biology. 84 (1): 41–45. doi:10.1007/BF00394525. ISSN 1432-1793.
- Quigg, Antonietta; Reinfelder, John R.; Fisher, Nicholas S. (2006). "Copper uptake kinetics in diverse marine phytoplankton". Limnology and Oceanography. 51 (2): 893–899. doi:10.4319/lo.2006.51.2.0893. ISSN 1939-5590.
- Brand, Larry E.; Sunda, William G.; Guillard, Robert R. L. (1986-05-01). "Reduction of marine phytoplankton reproduction rates by copper and cadmium". Journal of Experimental Marine Biology and Ecology. 96 (3): 225–250. doi:10.1016/0022-0981(86)90205-4. ISSN 0022-0981.
- Prociv P (September 2004). "Algal toxins or copper poisoning—revisiting the Palm Island "epidemic"". Med. J. Aust. 181 (6): 344. doi:10.5694/j.1326-5377.2004.tb06316.x. PMID 15377259. S2CID 22054004.