Dimanganese decacarbonyl
Dimanganese decacarbonyl is the chemical compound with the formula Mn2(CO)10. This metal carbonyl is an important reagent in the organometallic chemistry of manganese.[2]
10.png.webp) | |
 | |
| Names | |
|---|---|
| IUPAC name
bis(pentacarbonylmanganese)(Mn—Mn) | |
| Other names
Manganese carbonyl Decacarbonyldimanganese | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.392 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Mn2(CO)10 | |
| Molar mass | 389.98 g/mol |
| Appearance | Yellow crystals |
| Density | 1.750 g/cm3 |
| Melting point | 154 °C (309 °F; 427 K) |
| Boiling point | sublimes 60 °C (140 °F; 333 K) at 0.5 mm Hg |
| Insoluble | |
| Structure[1] | |
| monoclinic | |
a = 14.14 Å, b = 7.10 Å, c = 14.63 Å α = 90°, β = 105.2°, γ = 90° | |
Formula units (Z) |
4 |
| 0 D | |
| Hazards | |
| Main hazards | CO source |
| R-phrases (outdated) | 23/24/25 |
| S-phrases (outdated) | 22-26-36/37/39-45 |
| Related compounds | |
Related compounds |
Re2(CO)10 Co2(CO)8 Fe3(CO)12 Fe2(CO)9 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The compound was first prepared in low yield by the reduction of manganese iodide with magnesium under CO.[3] A more efficient preparation entails reduction of anhydrous MnCl2 with sodium benzophenone ketyl under 200 atmospheres of CO.[4] The availability of inexpensive methylcyclopentadienyl manganese tricarbonyl ("MMT") has led to a low pressure route to Mn2(CO)10.[5]
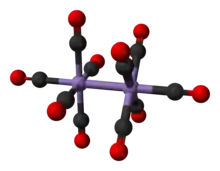
Structure
The crystal structure of Mn2(CO)10 was redetermined at high precision at room temperature in 1981[1] and bond lengths mentioned herein refer to results from that study. Mn2(CO)10 has no bridging CO ligands: it can be described as containing two axially-linked (CO)5Mn- subunits. There are two kinds of CO ligands; there is one CO linked to each Mn atom that is coaxial with the Mn-Mn bond and there are four “equatorial” carbonyls bonded to each Mn atom that are nearly perpendicular to the Mn-Mn bond (Mn’-Mn-CO(equatorial) angles range from 84.61(7) to 89.l6(7) degrees). The axial carbonyl distance of (181.1 pm) is 4.5 pm shorter than the average equatorial manganese-carbonyl distance of 185.6 pm. In the stable rotamer, the two Mn(CO)5 subunits are staggered. Thus, the overall molecule has approximate point group D4d symmetry, which is an uncommon symmetry shared with S2F10.[6] The Mn2(CO)10 molecule is isomorphous with Re2(CO)10 and the corresponding decacarbonyl of Tc.
Reactions
Mn2(CO)10 is air stable as a crystalline solid, but solutions require Schlenk techniques. It finds limited use in organic synthesis.[7] Characteristic reactions:
- Reduction of Mn2(CO)10 gives the manganese pentacarbonyl anion, which can be isolated as a salt:
- Mn2(CO)10 + 2 Na → 2 Na[Mn(CO)5]
The anion is a versatile nucleophile. Protonation gives the hydride [HMn(CO)5], and methylation gives [(CH3)Mn(CO)5].
- Bromination of Mn2(CO)10 proceeds with scission of the Mn-Mn bond to give manganese pentacarbonyl bromide.
- Mn2(CO)10 + Br2 → 2[Mn(CO)5Br]
Safety
Mn2(CO)10 is a volatile source of a metal and a source of CO.
References
- Melvyn Rowen Churchill, Kwame N. Amoh, and Harvey J. Wasserman. "Redetermination of the crystal structure of dimanganese decacarbonyl and determination of the crystal structure of dirhenium decacarbonyl. Revised values for the manganese-manganese and rhenium-rhenium bond lengths in dimanganese decacarbonyl and dirhenium decacarbonyl". Inorg. Chem. 20 (5): 1609–1611. doi:10.1021/ic50219a056.CS1 maint: multiple names: authors list (link)
- Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2
- Brimm, E. O.; Lynch, M. A.; Sesny, W. J. "Preparation and Properties of Manganese Carbonyl" Journal of the American Chemical Society 1954, volume 76, page 3831 - 3835.
- King, R. B. Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Press: New York, 1965. ISBN 0-444-42607-8
- King, R. B.; Stokes, J. C.; Korenowski, T. F. "A Convenient Synthesis of Dimanganese Decarbonyl from Inexpensive Starting Materials at Atmospheric Pressure" Journal of Organometallic Chemistry 1968, volume 11, Pages 641-643.
- L. F. Dahl, E. Ishishi, R. E. Rundle "Polynuclear Metal Carbonyls. I. Structures of Mn2(CO)10 and Re2(CO)10 J. Chem. Phys. 1957, volume 26, p. 1750. doi:10.1063/1.1743615
- Pauson, P. L. "Decacarbonyldimanganese" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rd001.pub2.