Enrofloxacin
Enrofloxacin (ENR) is a fluoroquinolone antibiotic sold by the Bayer Corporation under the trade name Baytril. Enrofloxacin is currently approved by the FDA for the treatment of individual pets and domestic animals in the United States. In September 2005, the FDA withdrew approval of Baytril for use in water to treat flocks of poultry, as this practice was noted to promote the evolution of fluoroquinolone-resistant strains of the bacterium Campylobacter, a human pathogen.[3]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, subcutaneous injection, topical (ear drops) |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% in dogs, 65-75% in sheep [1] |
| Metabolism | Renal and non-renal[1] |
| Elimination half-life | 4–5 hours in dogs, 6 hours in cats, 1.5 - 4.5 hours in sheep. |
| Excretion | Bile (70%); Renal (30%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.355 |
| Chemical and physical data | |
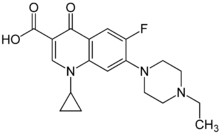
| Formula | C19H22FN3O3 |
| Molar mass | 359.4 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 219 to 221 °C (426 to 430 °F) |
| |
| |
| (verify) | |
It is a bactericidal agent. The bactericidal activity of enrofloxacin is concentration-dependent, with susceptible bacteria cell death occurring within 20–30 minutes of exposure. Enrofloxacin has demonstrated a significant post-antibiotic effect for both Gram-negative and Gram-positive bacteria and is active in both stationary and growth phases of bacterial replication. Enrofloxacin is partially deethylated by CYP450 into the active metabolite ciprofloxacin, which is also a fluoroquinolone antibiotic.
Activity and susceptibility data
Enrofloxacin is a synthetic antibacterial agent from the class of the fluoroquinolone carboxylic acid derivatives. It has antibacterial activity against a broad spectrum of Gram-negative and Gram-positive bacteria. It is effective against:
- Pseudomonas aeruginosa
- Klebsiella
- Escherichia coli
- Enterobacter
- Campylobacter
- Shigella
- Salmonella
- Aeromonas
- Haemophilus
- Proteus
- Yersinia
- Serratia
- Vibrio
- Brucella
- Chlamydia trachomatis
- Staphylococcus (including penicillinase-producing and methicillin-resistant strains)
- Mycoplasma
- Mycobacterium
Variable activity against:
Ineffective against:
The following data represent minimum inhibitory concentration ranges for a few medically significant bacterial pathogens:
- Escherichia coli - 0.022 - 0.03 µg/ml
- Staphylococcus aureus - 0.0925 - 64 µg/ml
- Pseudomonas aeruginosa - 0.05 µg/ml
Contraindications/precautions
Usage in poultry.
Adverse effects/warnings
Enrofloxacin was banned for poultry use in 2005.[4]
Overdosage/acute toxicity
It is unlikely that an acute overdose of either compound would result in symptoms more serious than either anorexia or vomiting, but the adverse effects noted above could occur. Dogs receiving 10 times the labeled dosage rate of enrofloxacin for at least 14 days developed only vomiting and anorexia. Death did occur in some dogs when fed 25 times the labeled rate for 11 days, however.
- Oral LD50: greater than 5000 mg/kg
- Dermal LD50: greater than 2000 mg/kg
- Inhalation LD50: greater than 3547 mg/m3 (4-hour exposure)
- Eye effects: irritant; reversible in less than 7 days. In cats, it can produce sudden onset blindness when administered by injection, as it is retinotoxic.
Degradation
The brown rot fungus Gloeophyllum striatum can degrade the fluoroquinolone enrofloxacin using hydroxyl radicals.[5]
References
- Plumb DC. "Enrofloxacin". Veterinary Drug Handbook (fifth ed.).
- "Baytril: Excretion and Elimination". Bayer HealthCare AG. Archived from the original on 2014-01-06. Retrieved 2014-01-06.
- "Enrofloxacin for Poultry". U.S. Food and Drug Administration. Archived from the original on 2007-02-10. Retrieved 2007-03-07.
- Morgan D, Kaufman M (April 30, 2005). "Lawmakers' Help for Drug Firm Tests Limits". Washington Post.
FDA Calls Efforts For Bayer Illegal
- Wetzstein HG, Schmeer N, Karl W (November 1997). "Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: identification of metabolites". Applied and Environmental Microbiology. 63 (11): 4272–81. doi:10.1128/AEM.63.11.4272-4281.1997. PMC 168747. PMID 9361414.