Ethenone
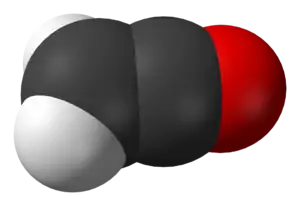
Ethenone is the formal name for ketene, an organic compound with formula C2H2O or H2C=C=O. It is the simplest member of the ketene class. It is a tautomer of the even less stable ethynol.
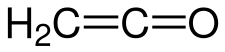 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethenone[1] | |
| Other names
Ketene Carbomethene Keto-ethylene | |
| Identifiers | |
3D model (JSmol) |
|
| 1098282 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.671 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H2O | |
| Molar mass | 42.037 g/mol |
| Appearance | Colourless gas |
| Odor | penetrating |
| Density | 1.93 g/cm3 |
| Melting point | −150.5 °C (−238.9 °F; 122.6 K) |
| Boiling point | −56.1 °C (−69.0 °F; 217.1 K) |
| decomposes | |
| Solubility | soluble in acetone ethanol ethyl ether aromatic solvents halocarbons |
| Vapor pressure | >1 atm (20°C)[2] |
Refractive index (nD) |
1.4355 |
| Thermochemistry | |
Heat capacity (C) |
51.75 J/K mol |
Std enthalpy of formation (ΔfH⦵298) |
-87.24 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | −107 °C (−161 °F; 166 K) |
| Explosive limits | 5.5-18% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1300 mg/kg (oral, rat) |
LC50 (median concentration) |
17 ppm (mouse, 10 min)[3] |
LCLo (lowest published) |
23 ppm (mouse, 30 min) 53 ppm (rabbit, 2 hr) 53 ppm (guinea pig, 2 hr) 750 ppm (cat, 10 min) 200 ppm (monkey, 10 min) 50 ppm (mouse, 10 min) 1000 ppm (rabbit, 10 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.5 ppm (0.9 mg/m3)[2] |
REL (Recommended) |
TWA 0.5 ppm (0.9 mg/m3) ST 1.5 ppm (3 mg/m3)[2] |
IDLH (Immediate danger) |
5 ppm[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Ethenone is a highly reactive gas (at standard conditions) and has a sharp irritating odour. It is only reasonably stable at low temperatures (−80 °C). It must therefore always be prepared for each use and processed immediately, otherwise a dimerization to diketene occurs or it reacts to polymers that are difficult to handle. The polymer content formed during the preparation is reduced, for example, by adding sulfur dioxide to the ketene gas.[4] Because of its cumulative double bonds, ethenone is highly reactive and reacts in an addition reaction H-acidic compounds to the corresponding acetic acid derivatives. It does for example react with water to acetic acid or with primary or secondary amines to the corresponding acetamides.
Ethenone is highly poisonous; its toxicity is about eight times that of phosgene.[5]
Ethenone tends to spontaneously polymerize. Contact with hydrogen peroxide leads to an explosive reaction. It can form an explosive mixture with air.
It is soluble in acetone, ethanol, ethyl ether, aromatic solvents and halocarbons.
Preparation
Ethenone was discovered at the same time by Hermann Staudinger (by reaction of bromoacetyl bromide with metallic zinc)[6][7]
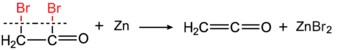
and by Norman T. M. Wilsmore (by thermal decomposition of acetic anhydride).[8]
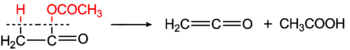
Ethenone is produced on a large scale industrially for use in the production of acetic anhydride. It can be prepared by pyrolysis of acetone, and this was formerly the main industrial process. When passing acetone vapors through heated pipes or electrically heated metal (like copper) wires at 500-600 °C in the presence of little carbon disulfide (CS2), acetone decomposes into methane and ethenone, with 95% yield.[9][10]

In industrial chemistry, ketone pyrolysis has largely been replaced by the dehydration of acetic acid (the Schmidlin-Bergman-Wilsmore reaction).[11]

Natural occurrence
Ethenone has been observed to occur in space, in comets or in gas as part of the interstellar medium.[12]
Use
Ethenone is used to make acetic anhydride from acetic acid. Generally it is used for the acetylation of chemical compounds.[5]
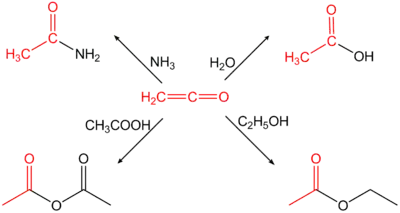 Reactions with acetamide, ethyl acetate, acetic acid and acetic anhydride
Reactions with acetamide, ethyl acetate, acetic acid and acetic anhydride
 Mechanism of the above reactions
Mechanism of the above reactions
Ethenone reacts with methanal in the presence of catalysts such as Lewis acids (AlCl3, ZnCl2 or BF3) to give β-propiolactone.[13] The technically most significant use of ethenone is the synthesis of sorbic acid by reaction with 2-butenal (crotonaldehyde) in toluene at about 50 °C in the presence of zinc salts of long-chain carboxylic acids. This produces a polyester of 3-hydroxy-4-hexenoic acid, which is thermally[14] or hydrolytically depolymerized to sorbic acid.
Ethenone is very reactive, tending to react with nucleophiles to form an acetyl group. For example, it reacts with water to form acetic acid;[15] with acetic acid to form acetic anhydride; with ammonia and amines to form ethanamides;[16] and with dry hydrogen halides to form acetyl halides.[17]
The formation of acetic acid likely occurs by an initial formation of 1,1-dihydroxyethene, which then tautomerizes to give the final product.[18]
Ethenone will also react with itself via [2 + 2] photocycloadditions to form cyclic dimers known as diketenes. For this reason, it should not be stored for long periods.[19]
Hazards
Exposure to concentrated levels causes humans to experience irritation of body parts such as the eye, nose, throat and lungs. Extended toxicity testing on mice, rats, guinea pigs and rabbits showed that ten-minute exposures to concentrations of freshly generated ethenone as low as 0.2 mg/liter (116 ppm) may produce a high percentage of deaths in small animals. These findings show ethenone is toxicologically identical to phosgene.[20]
The formation of ketene in the pyrolysis of vitamin E acetate, an additive of some e-liquid products, is one possible mechanism of the reported pulmonary damage[21] caused by electronic cigarette use.[22] A number of patents describe the catalytic formation of ketene from carboxylic acids and acetates, using a variety of metals or ceramics, some of which are known to occur in e-cigarette devices from patients with e-cigarette or vaping product-use associated lung injury (EVALI).[23][24]
Occupational exposure limits are set at 0.5 ppm (0.9 mg/m3) over an eight-hour time-weighted average.[25] An IDLH limit is set at 5 ppm, as this is the lowest concentration productive of a clinically relevant physiologic response in humans.[26]
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 723. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0367". National Institute for Occupational Safety and Health (NIOSH).
- "Ketene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- EP 0377438, R. Bergamin et al., issued 1990-06-11, assigned to Lonza AG
- Entry on Diketen. at: Römpp Online. Georg Thieme Verlag, retrieved 16. Juni 2014.
- H. Staudinger H. W. Klever (1908): "Keten. Bemerkung zur Abhandlung zur Abhandlung der HHrn. V.T. Wilsmore und A. W. Stewart". Berichte der deutschen chemischen Gesellschaft, volume 41, issue 1, pages 1516-1517. doi:10.1002/cber.190804101275
- Tidwell, T. T. (2005), "Ein Jahrhundert Ketene (1905–2005): die Entdeckung einer vielseitigen Klasse reaktiver Intermediate". Angewandte Chemie, volume 117, pages 5926–5933. doi:10.1002/ange.200500098
- Norman Thomas Mortimer Wilsmore (1907): "Keten". Journal of the Chemical Society, Transactions, volume 91, article CLXXXVIII (188), pages 1938-1941. doi:10.1039/ct9079101938
- K.-H. Lautenschläger, W. Schröter, A. Wanninger, "Taschenbuch der Chemie", 20. Aufl. 2006, ISBN 978-3-8171-1761-1.
- "Ketene". Organic Syntheses. doi:10.15227/orgsyn.004.0039.
- J. Schmidlin, M. Bergman (1910): Berichte der deutschen chemischen Gesellschaft, volume 43, pages 2821-. doi:10.1002/cber.19100430340.
- Hudson, Reggie L.; Loeffler, Mark J. (2013). "Ketene Formation in Interstellar Ices: A Laboratory Study". The Astrophysical Journal. 773 (2): 109. doi:10.1088/0004-637x/773/2/109. hdl:2060/20140010162. ISSN 0004-637X.
- Hans-Jürgen Arpe, "Industrielle Organische Chemie", 6. Aufl., 2007, WILEY-VCH Verlag, Weinheim, ISBN 978-3-527-31540-6.
- EP 1295860, D. Decker et al., issued 26. März 2003-03-26, assigned to Nutrinova GmbH
- Tidwell, p. 11.
- Tidwell, p. 560.
- ChemSpider http://www.chemspider.com/Chemical-Structure.9643.html
- Nguyen, Minh Tho; Raspoet, Greet (1999). "The hydration mechanism of ketene: 15 years later". Can. J. Chem. 77: 817–829. doi:10.1139/v99-090.
- Christoph Taeschler :Ketenes, Ketene Dimers, and Related Substances, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, New York, 2010
- H. A. Wooster; C. C. Lushbaugh; C. E. Redeman (1946). "The Inhalation Toxicity of Ketene and of Ketene Dimer". J. Am. Chem. Soc. 68 (12): 2743. doi:10.1021/ja01216a526.
- https://time.com/5753947/vaping-lung-disease-outbreak-peak/
- Dan Wu and F. O’Shea, "Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate", PNAS March 24, 2020 117 (12) 6349-6355.
- K. Attfield, W. Chen, K. Cummings, P. Jacob 3rd, D. O'Shea, J. Wagner, P. Wang, and J. Fowles, https://pubmed.ncbi.nlm.nih.gov/32551843/ Potential of ethenone (ketene) to contribute to e-cigarette, or vaping product use-associated lung injury. Am J Respir Crit Care Med, 2020 doi: 10.1164/rccm.202003-0654LE.
- U.S. patent No. 5475144. Catalyst and process for synthesis of ketenes from carboxylic acids. Dec 12, 1995. https://patents.google.com/patent/US5475144A/en
- Centers for Disease Control and Prevention (4 April 2013). "Ketene". NIOSH Pocket Guide to Chemical Hazards. Retrieved 13 November 2013.
- Centers for Disease Control and Prevention (May 1994). "Ketene". Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs). Retrieved 13 November 2013.
Literature
External links
 Media related to Ethenone at Wikimedia Commons
Media related to Ethenone at Wikimedia Commons

