Fibrocystic breast changes
Fibrocystic breast changes is a condition of the breasts where there may be pain, breast cysts, and breast masses.[1] The breasts may be described as "lumpy" or "doughy".[3] Symptoms may worsen during certain parts of the menstrual cycle.[1] It is not associated with cancer.[2]
| Fibrocystic breast changes | |
|---|---|
| Other names | Fibrocystic change, fibrocystic breast disease,[1] fibrocystic breast condition |
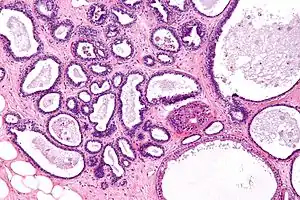 | |
| Micrograph showing fibrocystic breast changes. H&E stain. | |
| Specialty | Gynaecology |
| Symptoms | Breast pain, breast cysts, breast masses[2] |
| Usual onset | 30 to 50 years old[1] |
| Risk factors | Early age at first menstrual period, having children late or not having children[2] |
| Diagnostic method | Periodic examination, possibly medical imaging or breast biopsy[1] |
| Differential diagnosis | Breast cancer[1] |
| Treatment | Education about the condition, a well fitting bra, pain medication[1] |
| Prognosis | Good[1] |
| Frequency | Up to 60% of women[3] |
Risk factors include an early age at first menstrual period and either having children late or not having children.[2] It is not a disease but represents normal breast changes.[3] Diagnosis typically involves ruling out breast cancer.[1] Fibrocystic change includes fibroadenomas, fibrosis, and papillomas of the breast.[1]
Management may involve education about the condition, a well fitting bra, and pain medication.[1] Occasionally danazol or tamoxifen may be used for pain.[1] It is estimated that up to 60% of women are affected.[3] Women between the ages of 30 and 50 are most commonly affected.[1]
Signs and symptoms
The changes in fibrocystic breast disease are characterised by the appearance of fibrous tissue and a lumpy, cobblestone texture in the breasts. These lumps are smooth with defined edges, and are usually free-moving in regard to adjacent structures. The lumps can sometimes be obscured by irregularities in the breast that are associated with the condition. The lumps are most often found in the upper, outer sections of the breast (nearest to the armpit), but can be found throughout the breast. Women with fibrocystic changes may experience a persistent or intermittent breast aching or breast tenderness related to periodic swelling. Breasts and nipples may be tender or itchy.
Symptoms follow a periodic trend tied closely to the menstrual cycle. Symptoms tend to peak in the days and, in severe cases, weeks before each period and decrease afterwards. At peak, breasts may feel full, heavy, swollen, and tender to the touch. No complications related to breastfeeding have been found.
Pathophysiology
The exact mechanism of the condition is not fully understood, though it is known to be tied to hormone levels, as the condition usually subsides after menopause and is also related to the menstrual cycle. Post-menopausal women placed on hormone replacement therapy have also reported symptoms of fibrocystic breast change indicating hormones may play a role.
Fibrocystic breast changes is a cumulative process, caused partly by the normal hormonal variation during a woman's monthly cycle. The most important of these hormones are estrogen, progesterone and prolactin.
These hormones directly affect the breast tissues by causing cells to grow and multiply.[4] Many other hormones such as TSH, insulin, growth hormone and growth factors such as TGF-beta exert direct and indirect effects amplifying or regulating cell growth. Years of such fluctuations eventually produce small cysts and/or areas of dense or fibrotic tissue. Multiple small cysts and an increasing level of breast pain commonly develop when a woman hits her 30s. Larger cysts usually do not occur until after the age of 35.[5] Over time, presumably driven by aberrant growth signals, such lesions may accumulate epigenetic, genetic and karyotypic changes such as modified expression of hormone receptors and loss of heterozygosity.
Several variants of fibrocystic breast changes may be distinguished and these may have different causes and genetic predispositions. Adenosis involves abnormal count and density of lobular units, while other lesions appear to stem mainly from ductal epithelial origins.
There is preliminary evidence that iodine deficiency contributes to fibrocystic breast changes by enhancing the sensitivity of breast tissue to estrogen.[6][7][8][9]
Diagnosis
Diagnosis is mostly done based on symptoms after exclusion of breast cancer. Nipple fluid aspiration can be used to classify cyst type (and to some extent improve breast cancer risk prediction) but it is rarely used in practice. Biopsy or fine needle aspiration are rarely warranted.[10]
Fibrocystic breast disease is primarily diagnosed based on the symptoms, clinical breast exam and on a physical exam. During this examination, the doctor checks for unusual areas in the breasts, both visually and manually. Also, the lymph nodes in the axilla area and lower neck are examined. A complete and accurate medical history is also helpful in diagnosing this condition. If the patient's medical history and physical exam findings are consistent with normal breast changes, no additional tests are considered but otherwise the patient will be asked to return a few weeks later for reassessment.[11] Women may detect lumps in their breasts during self-examination as well.
Imaging
In order to establish whether the lump is a cyst or not, several imaging tests may be performed. Mammography is usually the first imaging test to be ordered when unusual breast changes have been detected during a physical examination. A diagnostic mammography consists in a series of X-rays that provide clear images of specific areas of the breast.
Ultrasounds and MRIs are commonly performed in conjunction with mammographies as they produce clear images of the breast and clearly distinguish between fluid-filled breast cysts and solid masses. The ultrasound and MRI exams can better evaluate dense tissue of the breast; hence it is often undergone by young patients, under 30 years old.
Biopsy
The breast biopsy is usually the test used to confirm the suspected diagnosing. After imaging tests have been performed and have revealed unusual areas or lumps in the breast, a breast biopsy will be ordered. This test consists in removing a sample of breast tissue which is then looked at under a microscope. The specialist analyzing the tissue sample will be able to conclude if the breast changes are benign or malignant or whether breast fibrocystic disease is present.
There are four main types of breast biopsies that may be performed. A fine-needle aspiration biopsy is usually ordered when the doctor is almost certain that the lump is a cyst. This test is generally performed in conjunction with an ultrasound which is helpful in guiding the needle into a small or hard to find lump. The procedure is painless and it consists in inserting a thin needle into the breast tissue while the lump is palpated.
The core-needle biopsy is normally performed under local anesthesia and in a physician's office. The needle used in this procedure is slightly larger than the one used for a fine-needle biopsy because the procedure is intended to remove a small cylinder of tissue that will be sent to the laboratory for further examination.
A newer type of breast biopsy is the stereotactic biopsy that relies on a three-dimensional X-ray to guide the needle biopsy of non-palpable mass. The biopsy is performed in a similar manner, by using a needle to remove tissue sample but locating the specific area of the breast is done by X-raying the breast by two different angles. Surgical biopsy is a procedure performed to remove the entire lump or a part of it for laboratory analyzing. It may be painful and is done under local anesthesia.
Treatment
Most women with fibrocystic changes and no symptoms do not need treatment, but closer follow-up may be advised.[12]
There is no widely accepted treatment or prevention strategy for fibrocystic condition. When treatment of symptoms is necessary it follows the same strategies as treatment for cyclical breast pain.
It is controversial whether benign breast conditions improve or worsen with oral contraceptives or hormone replacement therapy.[13]
A few small-scale studies have indicated that the fibrocystic condition may be improved by dietary changes (especially by a reduced intake of caffeine and related methylxanthines or by a reduced intake of salt) and by vitamin supplements.[14]
Tentative evidence shown beneficial effects of iodine supplementation in women with fibrocystic breast changes.[6][15][16]
Prognosis
There are usually no adverse side effects to this condition. In almost all cases it subsides after menopause. A possible complication arises through the fact that cancerous tumors may be more difficult to detect in women with fibrocystic changes.
Breast cancer risk
Breast cancer risk is elevated for defined fraction of lesions. Except for people with a strong family history of breast cancer, where the risk is two-fold, nonproliferative lesions have no increased risk. Proliferative lesions also have approximately a 2-fold risk. In particular, atypical hyperplasia is associated with an increased risk of developing breast cancer.[17] Atypical lobular hyperplasia is associated with the greatest risk, approximately 5-fold and especially high relative risk of developing premenopausal breast cancer. Atypical ductal hyperplasia is associated with 2.4-fold risk.[18] In contrast, a New England Journal of Medicine article [19] states that for women with a strong familial history of breast cancer, the risk of future breast cancer is roughly doubled, independent of histological status. The article further states "The relative risk of breast cancer for the cohort was 1.56 (95 percent confidence interval, 1.45 to 1.68), and this increased risk persisted for at least 25 years after biopsy. The relative risk associated with atypia was 4.24 (95 percent confidence interval, 3.26 to 5.41), as compared with a relative risk of 1.88 (95 percent confidence interval, 1.66 to 2.12) for proliferative changes without atypia and of 1.27 (95 percent confidence interval, 1.15 to 1.41) for nonproliferative lesions. The strength of the family history of breast cancer, available for 4808 women, was a risk factor that was independent of histologic findings. No increased risk was found among women with no family history and nonproliferative findings. In the first 10 years after the initial biopsy, an excess of cancers occurred in the same breast, especially in women with atypia."
It is not well understood whether the lesions are precursors of breast cancer or only indication of increased risk, for most types of lesions the chance of developing breast cancer is nearly the same in the affected and unaffected breast (side) indicating only coincidence of risk factors. For atypical lobular hyperplasia there is high incidence of ipsilateral breast cancers indicating a possible direct carcinogenetic link.[20]
Epidemiology
The estimated figures for the prevalence of fibrocystic breast changes in women over lifetime vary widely in the literature, with estimates ranging from about 30 to 60 %[21] over about 50 to 60 %[22] to about 60 to 75% of all women.[23]
The condition is most common among women between 30 and 50 years of age.[23]
Terminology
In ICD-10 the condition is called diffuse cystic mastopathy, or, if there is epithelial proliferation, fibrosclerosis of breast.[24] Other names for this condition include chronic cystic mastitis, fibrocystic mastopathy and mammary dysplasia.[25] The condition has also been named after several people (see eponyms below). Since it is a very common disorder, some authors have argued that it should not be termed a "disease",[26] whereas others feel that it meets the criteria for a disease. It is not a classic form of mastitis (breast inflammation).[27]
Eponyms
This entity has historically also been termed Bloodgood’s disease, Cooper's disease (after Sir Astley Paston Cooper, 1st baronet), Phocas' disease, Reclus’ disease and Reclus’ syndrome (after Paul Reclus), Reclus-Schimmelbusch disease, Schimmelbusch disease and Tillaux-Phocas disease.[28]
References
- Ferri, Fred F. (2018). Ferri's Clinical Advisor 2019: 5 Books in 1. Elsevier Health Sciences. p. 548. ISBN 9780323550765.
- "Breast Masses (Breast Lumps)". Merck Manuals Professional Edition. Retrieved 3 November 2018.
- Santen, RJ; Mansel, R (21 July 2005). "Benign breast disorders". The New England Journal of Medicine. 353 (3): 275–85. doi:10.1056/NEJMra035692. PMID 16034013.
- "Fibrocystic Breast Condition".2010/04/13, MedicineNet.com
- "Fibrocystic Breast Condition".2010/04/13
- Cann, Stephen A.; van Netten, Johannes P.; van Netten, Christiaan (2000). "Hypothesis: iodine, selenium and the development of breast cancer". Cancer Causes and Control (review). 11 (2): 121–127. doi:10.1023/A:1008925301459. ISSN 0957-5243. PMID 10710195. S2CID 2665461.
- Joseph E. Pizzorno; Michael T. Murray (14 September 2012). Textbook of Natural Medicine. Elsevier Health Sciences. p. 1371. ISBN 978-1-4377-2333-5.
- Venturi, S. (2001). "Is there a role for iodine in breast diseases?". The Breast. 10 (5): 379–382. doi:10.1054/brst.2000.0267. PMID 14965610.
- Aceves, C.; Anguiano, B.; Delgado, G. (2005). "Is iodine a gatekeeper of the integrity of the mammary gland?". Journal of Mammary Gland Biology and Neoplasia. 10 (2): 189–196. doi:10.1007/s10911-005-5401-5. PMID 16025225. S2CID 16838840.
- Vaidyanathan, L.; Barnard, K.; Elnicki, D M. (May 2002). "Benign Breast Disease: When To Treat, When To Reassure, When To Refer". Cleveland Clinic Journal of Medicine. 69 (5): 424–439. doi:10.3949/ccjm.69.5.425. PMID 12022387.
- "Tests and diagnosis". Archived from the original on 2010-03-22.2010/04/13
- "Types of non-cancerous breast conditions".2010/04/13
- Gadducci A, Guerrieri ME, Genazzani AR (February 2012). "Benign breast diseases, contraception and hormone replacement therapy". Minerva Ginecologica. 64 (1): 67–74. PMID 22334232.
- Ethel Sloane, Biology of Women, Cengage Learning, 2002, p. 200-201
- "Iodine: Fact Sheet for Health Professionals". NIH. Retrieved 2015-02-07.
- Kessler JH (2004). "The effect of supraphysiologic levels of iodine on patients with cyclic mastalgia". The Breast Journal (Randomized Controlled Trial). 10 (4): 328–36. doi:10.1111/j.1075-122X.2004.21341.x. PMID 15239792. S2CID 2685253.
- Ethel Sloane, Biology of Women, Cengage Learning, 2002, p. 200
- Marshall, LM; Hunter, DJ; Connolly, JL; Schnitt, SJ; Byrne, C; London, SJ; Colditz, GA (1997). "Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types". Cancer Epidemiology, Biomarkers & Prevention. 6 (5): 297–301. PMID 9149887.
- Hartmann, L. C.; Sellers, T. A.; Frost, M. H.; Lingle, W. L.; Degnim, A. C.; Ghosh, K; Vierkant, R. A.; Maloney, S. D.; Pankratz, V. S.; Hillman, D. W.; Suman, V. J.; Johnson, J; Blake, C; Tlsty, T; Vachon, C. M.; Melton Lj, 3rd; Visscher, D. W. (2005). "Benign breast disease and the risk of breast cancer". New England Journal of Medicine. 353 (3): 229–37. doi:10.1056/NEJMoa044383. PMID 16034008.
- Page, D. L.; Schuyler, P. A.; Dupont, W. D.; Jensen, R. A.; Plummer Jr, W. D.; Simpson, J. F. (2003). "Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study". The Lancet. 361 (9352): 125–9. doi:10.1016/S0140-6736(03)12230-1. PMID 12531579. S2CID 6429291.
- Susan L. Norwoord (March 1990). "Fibrocystic Breast Disease An Update and Review". JOGNN – Journal of Obstetric Gynecologic, & Neonatal Nursing. 19 (2): 116–121. doi:10.1111/j.1552-6909.1990.tb01629.x. PMID 2181087.
- Kelly A. McGarry; Iris L. Tong (6 July 2012). The 5-Minute Consult Clinical Companion to Women's Health. Lippincott Williams & Wilkins. p. 86. ISBN 978-1-4511-1654-0.
- Roger P. Smith (1 December 2008). Netter's Obstetrics and Gynecology. Elsevier Health Sciences. p. 371. ISBN 978-1-4377-2137-9.
- Disorders of breast (N60-N64) in ICD-10.
- Atlantic Women’s Specialists. "Fibrocystic Breast Changes". Retrieved 21 June 2012.
- Santen RJ, Mansel R (July 2005). "Benign breast disorders". N. Engl. J. Med. 353 (3): 275–85. doi:10.1056/NEJMra035692. PMID 16034013.
- Gokhale, Sudheer (August 2009). "Ultrasound Characterization of Breast Masses". Indian Journal of Radiology and Imaging. 19 (3): 242–247. doi:10.4103/0971-3026.54878. PMC 2766883. PMID 19881096.
- synd/1891 at Who Named It?
External links
| Classification | |
|---|---|
| External resources |