Fumed silica
Fumed silica (CAS number 112945-52-5), also known as pyrogenic silica because it is produced in a flame, consists of microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles. The resulting powder has an extremely low bulk density and high surface area. Its three-dimensional structure results in viscosity-increasing, thixotropic behavior when used as a thickener or reinforcing filler.[1]
Properties
Fumed silica has a very strong thickening effect. Primary particle size is 5–50 nm. The particles are non-porous and have a surface area of 50–600 m2/g. The density is 160–190 kg/m3.
Production
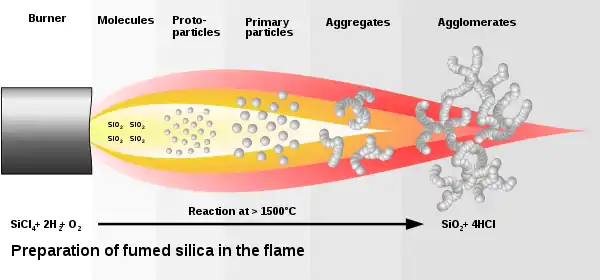
Fumed silica is made from flame pyrolysis of silicon tetrachloride or from quartz sand vaporized in a 3000 °C electric arc.[2] Major global producers are Evonik (who sells it under the name Aerosil), Cabot Corporation (Cab-O-Sil), Wacker Chemie (HDK), Dow Corning, Heraeus (Zandosil), Tokuyama Corporation (Reolosil), OCI (Konasil), Orisil (Orisil) and Xunyuchem(XYSIL).[3]
Applications
Fumed silica serves as a universal thickening agent and an anticaking agent (free-flow agent) in powders. Like silica gel, it serves as a desiccant. It is used in cosmetics for its light-diffusing properties. It is used as a light abrasive, in products like toothpaste. Other uses include filler in silicone elastomer and viscosity adjustment in paints, coatings, printing inks, adhesives and unsaturated polyester resins.[4]
It is also used in the production of cat litter box filler and as a core material in the production of vacuum insulated panels. Moreover, Fumed silica nanoparticles can potentially be used as efficient anti-aging coatings in asphalt pavements.[5]
Health issues
Fumed silica is not listed as a carcinogen by OSHA, IARC, or NTP. Due to its fineness and thinness, fumed silica can easily become airborne, making it an inhalation risk, capable of causing irritation.
See also
References
- Flörke, Otto W.; Graetsch, Heribert A.; Brunk, Fred; Benda, Leopold; Paschen, Siegfried; Bergna, Horacio E.; Roberts, William O.; Welsh, William A.; Libanati, Cristian; Ettlinger, Manfred; Kerner, Dieter; Maier, Monika; Meon, Walter; Schmoll, Ralf; Gies, Hermann; Schiffmann, Dietmar (15 April 2008). "Silica". Ullmann's Encyclopedia of Industrial Chemistry: a23_583.pub3. doi:10.1002/14356007.a23_583.pub3. ISBN 978-3527306732.
- Garrett, P.R. (1992). Defoaming. Theory and Industrial applications. USA: CRC Press. pp. 239–240. ISBN 0-8247-8770-6.
- Fumed Silica Manufacturer Overview
- "Reade Fumed Silica Powder (SiO2)". Fumed Silica Powder (SiO2). reade.com. Retrieved 27 January 2021.
- Cheraghian, Goshtasp; Wistuba, Michael P. (8 July 2020). "Ultraviolet aging study on bitumen modified by a composite of clay and fumed silica nanoparticles". Scientific Reports. 10 (1): 11216. Bibcode:2020NatSR..1011216C. doi:10.1038/s41598-020-68007-0. PMC 7343882. PMID 32641741.
