Haemoproteus
Haemoproteus is a genus of alveolates that are parasitic in birds, reptiles and amphibians. Its name is derived from Greek: Haima, "blood", and Proteus, a sea god who had the power of assuming different shapes. The name Haemoproteus was first used in the description of Haemoproteus columbae in the blood of the pigeon Columba livia by Kruse in 1890. This was also the first description of this genus. Two other genera — Halteridium and Simondia — are now considered to be synonyms of Haemoproteus.
| Haemoproteus | |
|---|---|
 | |
| Haemoproteus syrnii | |
| Scientific classification | |
| (unranked): | Diaphoretickes |
| Clade: | TSAR |
| Clade: | SAR |
| Infrakingdom: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Chromatorida |
| Suborder: | Laveraniina |
| Family: | Haemoproteidae |
| Genus: | Haemoproteus Kruse, 1890 |
| Species | |
|
| |
The protozoa are intracellular parasites that infect the erythrocytes. They are transmitted by blood sucking insects including mosquitoes, biting midges (Culicoides), louse flies (Hippoboscidae) and tabanid flies (Tabanidae). Infection with this genus is sometimes known as pseudomalaria because of the parasites' similarities with Plasmodium species.
Within the genus there are at least 173 species, 5 varieties and 1 subspecies. Of these over 140 occur in birds, 16 in reptiles and 3 in amphibia: 14 orders and 50 families of birds are represented. These include gamebirds (Galliformes), waterfowl (Anseriformes), raptors (Accipitriformes, Falconiformes, Strigiformes), pigeons and doves (Columbiformes), and perching birds or songbirds (Passeriformes).
Taxonomy and systematics
Evolution
The earliest known fossil is of a Haemoproteus like organism (Paleohaemoproteus burmacis) was found in the abdominal cavity of a female biting midge trapped 100 million years ago in amber found in Myanmar.[1]
Taxonomic history
The first description of this genus was in 1890 by Kruse who described Haemoproteus columbae in the blood of the pigeon Columba livia. McCallum in 1897 showed that the process of exflagellation was part of sexual reproduction in these parasites and thought it probable that the same process occurred in Plasmodium. The first record of a haemoproteid parasite in a reptile was by Simond in 1901 who gave it the name Haemamoeba metchnikovi. The Sergent brothers in 1906 showed that the ectoparasitic fly Pseudolynchia canariensis was the vector of Haemoproteus columbae. Aragao in 1908 demonstrated the schizogonic stages of Haemoproteus columbae in the endothelial cells of the lungs of nestling pigeons infected by the bite of infected Pseudolynchia. It was generally believed that transmission of the parasites was by regurgitation during a blood meal until Adie showed that the parasites develop in the salivary glands in a fashion analogous to that of Plasmodium in mosquitoes.
The genus Halterium was created by the French parasitologist Alphonse Labbe for a species he observed with gametocytes in erythrocytes, with pigment granules, and halter-shaped when fully formed. This genus was soon subsumed into the genus Haemoproteus.
The genus Haemocystidium was created to give a name to the haemoproteid of a gecko belonging to the genus Hemidactylus in Sri Lanka by Castellani and Willey in 1904. A second species in this genus was described in 1909 by Johnston and Cleland who found pigmented gametocytes in the blood of the Australian tortoise Chelodina longicollis. These species were transferred to Haemoproteus in 1926 by Wenyon.
The genus was resurrected by Garnham in 1966 when he created a new generic name — Simondia — for the haemoproteids of chelonians. He followed the opinions of Wenyon, Hewitt and DeGiusti and suggested that all these parasites belonged to the one species — Simondia metchnikovi. He retained the name Haemocystidium for the haemoproteids of lizards.
A different genus of vectors was identified in 1957 by Fallis and Wood when they identified Haemoproreus nettionis in Culicoides downesi Wirth and Hubert in Ontario, Canada.
Levine and Campbell in 1971 moved all the species in Simondia and Haemocystidium into Haemoproteus an opinion that was followed by subsequent authors.
The genus Haemocystidium was resurrected again by Telford in 1996 when he described three new species of protozoa in geckos from Pakistan.[2]
This genus like those of many protozoa may be further modified once additional DNA sequences are available. For instance, many DNA sequences have been identified for Haemoproteus in birds around the world in recent years, leading to new knowledge about the previously unknown diversity of this parasite in different regions[3]
Subgenera
The species infecting avian hosts have been divided into two subgenera — Haemoproteus and Parahaemoproteus — a division proposed in 1965 by Bennett et al. These may be distinguished as follows:
Haemoproteus: Vectors are hippoboscid flies (Hippoboscidae). Exflagellation does not occur below 20 degrees Celsius. Mature oocysts have diameters greater than 20 micrometres. The average length of the sporozoites is less than 10 micrometres. One end of the sporozoite is more pointed than the other. Although the majority are parasites of the Columbiformes, some species from this subgenus have also been reported in the Charadriiformes, Pelecaniformes and Suliformes.
Parahaemoproteus: Parasites of birds other than the Columbiformes. Vectors are biting midges (Ceratopogonidae). Exflagellation occurs below 20 degrees Celsius. Mature oocysts have diameters less than 20 micrometres. The average length of the sporozoites is greater than 10 micrometres. Both ends of the sporozoite are equally pointed.
While it was thought that Haemoproteus was limited to doves and related species, species in this genus have been isolated from frigatebirds.[4]
Species list
|
|
|
Life cycle
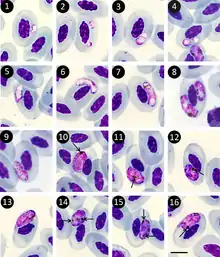
The infective stage is the sporozoite which is present in the salivary glands of the vector. Once the vector bites a new host, the sporozoites enter the blood stream and invade endothelial cells of blood vessels within various tissues including those of the lung, liver and spleen. Within the endothelial cells, the sporozoites undergo asexual reproduction becoming schizonts. These in turn produce numerous merozoites which penetrate the erythrocytes and mature into either female gametocytes (macrogametocytes) or male gametocytes (microgametocytes). Gametocytes can then be ingested by another blood-sucking insect where they undergo sexual reproduction in the midgut of the insect to produce oocysts. The oocysts rupture and release numerous sporozoites that invade the salivary gland and serve as a focus of subsequent infection for another host once the insect takes its next blood meal.
Description
Only gametocytes are found in the blood. Asexual reproduction occurs in body organs especially the liver. The organisms occupy the majority of the cytoplasm, leaving the light magenta, finely granular, pink nucleus centrally located.
Taxonomy of this genus is difficult as there are few distinct morphological differences between the recognised species. Many of them were described under the 'one species-one host' hypothesis which is now thought to be potentially misleading. The morphological features most commonly used to describe a species include the number of pigment granules, the degree of encirclement of the host nucleus, the size of the parasite, the degree of host nucleus displacement and the degree of host cell enlargement. DNA studies should help to clarify this area but to date have rarely been undertaken.
The gametocytes have five basic forms
- thin gametocytes with incomplete margins (H. balearicae, H. pelouri)
- halterial gametocytes (H. maccullumi)
- thick sausage shaped gametocytes that fill most of the host cell and displace the host nucleus laterally (H. halyconis, H. plataleae)
- gametocytes that encircle the host nucleus and fill the host cell (H. telfordi)
- straight gametocytes that normally occur in anucleate cells and are almost as long as the host cell (H. enucleator)
Diagnostic criteria
- Gametocytes are only present within erythrocytes
- Gametocytes have a "halter-shaped" appearance with little displacement of the host nucleus
- Schizonts are not seen on peripheral blood smears
- Multiple pigment granules (hemozoin) are present within the erythrocytes
Pigment granules are refractile and yellow to brown in colour.
Pathology
Infections with most Haemoproteus species appear to produce subclinical infections.
Post-mortem findings include enlargement of the spleen, liver and kidneys. These organs may appear chocolate-brown due to hemozoin deposition. Cytologic imprints may reveal schizont-laden endothelial cells. Some species of Haemoproteus will also form large, cyst-like bodies within the skeletal muscles that resembling those seen with Sarcocystis species infections.
Pigeons infected with Haemoproteus columbae may develop enlarged gizzards; and anemia has been recorded.[5]
Flocks of bobwhite quail (Colinus virginianus) may become infected with Haemoproteus lophortyx. Infected birds may suffer from reluctance to move, ruffled appearance, prostration and death. Other findings include parasitemia and anemia. Large megaloschizonts may be present in skeletal muscles, particularly those of the thighs and back. The average cumulative mortality for flocks experiencing outbreaks may be over 20%.
Experimental infection of turkeys with Haemoproteus meleagridis resulted in lameness, diarrhea, depression, emaciation, anorexia and occasionally anemia.
Muscovey ducks infected with Haemoproteus nettionis suffered lameness, dyspnea and sudden death.
In other avian species, anemia and anorexia have been reported occasionally. Importantly, new records of Haemoproteus are discovered constantly and should still be monitored for effects on host condition[6]
Effect on vectors
H. columbae infects rock pigeons (Columba livia) and is vectored by a hippoboscid fly (Pseudolynchia canariensis).[7] Both sexes of vector can transmit the parasite. Species of the Hippoboscoidea the superfamily to which Ps. canariensis belongs do not lay eggs. Instead the larvae hatch in utero, are fed internally by 'milk glands' and pass through three morphological stages before being deposited to pupate. The survival of female flies is significantly reduced when they were infected with the parasite. In contrast no effect is seen on male fly survival. Additionally the females produce fewer offspring when infected but the quality of the offspring does not seem to be affected.
Host records
Avian hosts
- H. anthi — yellow wagtails (Motacilla flava)
- H. antigonis — Florida sandhill crane (Grus canadensis pratensis)
- H. balearicae — black crowned crane (Balearica pavonina gibbericeps, Balearica pavonina pavonina), Florida sandhill crane (Grus canadensis pratensis)
- H. bambusicolae — bamboo partridge (Bambusicola thoracica sonorivox)
- H. beckeri — gray catbird (Dumetella carolinensis)
- H. (Parahaemoproteus) belopolskyi — blackcaps (Sylvia atricapilla)
- H. bennetti — greater yellownape (Picus flavinucha)
- H. borgesi — red cockaded woodpecker (Picoides borealis)
- H. brachiatus — saker falcon (Falco cherrug)
- H. bucerotis — red billed hornbill (Tockus erythrorhynchus)
- H. (Parahaemoproteus) canachites — grouse
- H. (Parahaemoproteus) catharti — turkey vulture (Cathartes aura)
- H. (Parahaemoproteus) coatneyi — bananaquit (Coereba flaveola), white-crowned sparrows (Zonotrichia leucophrys)
- H. (Haemoproteus) columbae — Japanese black wood pigeons (Columba janthina), pigeon (Columba livia), doves (Columbina talpacoti, Scardafella squammata, Zenaida auriculata), laughing dove (Stigmatopelia senegalensis), eastern white-winged doves (Zenaida asiatica asiatica), mourning doves (Zenaida macroura)
- H. (Parahaemoproteus) concavocentralis — hawfinch (Coccothraustes coccothraustes)
- H. cornuata — coppersmith barbet (Megalaima haemacephala)
- H. crumenium — wood stork (Mycteria americana)
- H. (Parahaemoproteus) cyanomitrae — olive sunbird (Cyanomitra olivacea)[8]
- H. (Parahaemoproteus) danilewskyi — blue jays (Cyanocitta cristata)
- H. (Parahaemoproteus) desseri — blossom headed parakeet (Psittacula roseata)
- H. dicruri — fork tailed drongo (Dicrurus adsimilis), crested drongos (Dicrurus forficatus)
- H. elani — Cooper's hawk (Accipiter cooperii), sharp shinned hawk (Accipiter striatus)
- H. enucleator — kingfisher (Ispidina picta)
- H. (Parahaemoproteus) fringillae — rufous-winged (Aimophila carpalis), house finch (Carpodacus mexicanus), hawfinch (Coccothraustes coccothraustes), oriental magpie robin (Copsychus saularis), dark-eyed juncos (Junco hyemalis), American redstarts (Setophaga ruticilla)
- H. forresteri — rufous-headed ground-roller (Atelornis crossleyi)
- H. gabaldoni — Muscovy duck (Cairina moschata)
- H. (Parahaemoproteus) garnhami — sparrows
- H. goodmani — pitta-like ground-roller (Atelornis pittoides)
- H. greineri — wood ducks (Aix sponsa), common mergansers (Mergus merganser), common pochard (Aythya ferina)[9]
- H. handai — lesser sulphur-crested cockatoo (Cacatua sulphurea), plum-headed parakeet (Psittacula cyanocephala), ring necked parakeet (Psittacula krameri manillensis)
- H. himalayanus — rufous sibia (Heterophasia capistrata)
- H. (Parahaemoproteus) homobelopolskyi — red headed malimbe (Malimbus rubricollis), black headed weaver (Ploceus melanocephalus), red billed quelea (Quelea quelea)
- H. (Parahaemoproteus) homopalloris - wood warblers (Phylloscopus sibilatrix)
- H. (Parahaemoproteus) homovelans — grey-faced woodpecker (Picus canus)
- H. (Parahaemoproteus) hudaidensis — blue checked bee-eater (Merops superciliosus persicus Pallas)
- H. ilanpapernai — spotted wood owl (Strix seloputo), Brown Hawk-Owl (Ninox scutulata)[10]
- H. iwa — great frigatebirds (Fregata minor), magnificent frigatebirds (Fregata magnificens)
- H. janovyi — whitebacked vulture (Gyps africanus), hooded vulture (Necrosyrtes monachus), white-headed vulture (Trigonoceps occipitalis) lappet faced vulture (Torgos tracheliotus)
- H. (Haemoproteus) jenniae — swallow tailed gull (Creagrus furcatus)
- H. khani — crested drongos (Dicrurus forficatus)
- H. (Parahaemoproteus) lanii — red backed shrike (Lanius collurio), woodchat shrike (Lanius senator)
- H. lari — Caspian gulls (Larus cachinnans)
- H. (Parahaemoproteus) lophortyx — California quail (Callipepla californica), scaled quail (Callipepla squamata), bobwhite quail (Colinus virginianus)
- H. maccallumi — mourning doves (Zenaida macroura)
- H. macrovacuolatus — black-bellied whistling duck (Dendrocygna autumnalis)
- H. madagascariensis — hook billed vanga (Vanga curvirostris)
- H. majoris — Swainson's thrush (Catharus ustulatus), blue tits (Cyanistes caeruleus, Parus caeruleus)
- H. mansoni — blue grouse (Dendragapus obscurus), ptarmigan (Lagopus lagopus)
- H. meleagridis — turkey (Meleagris gallopavo)
- H. (Parahaemoproteus) micronuclearis — red headed malimbe (Malimbus rubricollis), black headed weaver (Ploceus melanocephalus), red billed quelea (Quelea quelea)
- H. (Haemoproteus) multipigmentatus — Galapagos dove (Zenaida galapagoensis)
- H. motacillae — yellow wagtails (Motacilla flava)
- H. (Haemoproteus) multivolutinus — tambourine dove (Turtur timpanistria)
- H. (Parahaemoproteus) nettionis — wood ducks (Aix sponsa), blue-winged teals (Anas discors), Pekin duck (Anas platyrhynchos), lesser scaups (Aythya affinis), common pochard (Aythya ferina),[9] ring-necked ducks (Aythya collaris), Muscovey duck (Cairina moschata), trumpeter swans (Cygnus buccinator)
- H. nisi — Cooper's hawk (Accipiter cooperii), sharp shinned hawk (Accipiter striatus)
- H. (Parahaemoproteus) nucleofascialis — red headed malimbe (Malimbus rubricollis), black headed weaver (Ploceus melanocephalus), red billed quelea (Quelea quelea)
- H. noctuae — snowy owls (Nyctea scandiaca), spotted owl (Strix occidentalis)
- H. orioli — golden oriole (Oriolus oriolus)
- H. oryzivorae — oriental magpie robin (Copsychus saularis), Indian silverbill (Lonchura malabarica), tricoloured munia (Lonchura malacca ruboniger), scaly-breasted munia (Lonchura punctulata), baya weaver (Ploceus philippinus), jungle babbler (Turdoides striata)
- H. palumbus — pigeon (Columba palumbus palumbus)
- H. pallidulus — blackcap (Sylvia atricapilla)[11]
- H. parabelopolskyi — blackcap (Sylvia atricapilla)
- H. (Haemoproteus) paramultipigmentatus — Socorro common ground dove (Columbina passerina socorroensis)
- H. passeris — Israeli house sparrow (Passer domesticus biblicus)
- H. pasteris — pied myna (Sturnus contra), grey headed myna (Sturnus malabaricus)
- H. pastoris — greater blue eared glossy starling (Lamprotornis chalybaeus), pied myna (Sturnus contra)
- H. payevskyi — great reed warbler (Acrocephalus arundinaceus), marsh warbler (Acrocephalus palustris)
- H. (Haemoproteus) piresi — pigeon (Columba livia)
- H. plataleae — glossy ibis (Plegadis falcinellus)
- H. pratosi — Ahanta francolin (Francolinus ahantensis)
- H. pratasi — helmeted guineafowl (Numida meleagris)
- H. prognei — purple martin (Progne subis)
- H. psittaci — African grey parrot (Psittacus erithacus)
- H. raymundi — eastern olive sunbird (Nectarinia olivacea)
- H. (Haemoproteus) sacharovi — eastern white-winged doves (Zenaida asiatica asiatica), mourning doves (Zenaida macroura)
- H. sangunis — red whiskered bulbul (Pycnonotus jocosus emeria)
- H. (Parahaemoproteus) sanîosdiasï — chicken (Gallus gallus)
- H. silvaï — guinea fowl (Numida meleagris mitrata)
- H. sylvae — great reed warbler (Acrocephalus arundinaceus)
- H. syrnii — tawny owl (Strix aluco), spotted owl (Strix occidentalis), European scops owl (Otus scops)[12]
- H. telfordi — MacQueen's bustards (Chlamydotis macqueenii), rufous-crested bustards (Eupodotis ruficrista), great bustard (Otis tarda)
- H. tendeiroi — MacQueen's bustards (Chlamydotis macqueenii), rufous-crested bustards (Eupodotis ruficrista), great bustard (Otis tarda)
- H. tinnunculi — American kestrel (Falco sparverius), Chimango caracara (Milvago chimango)
- H. (Haemoproteus) turtur — turtle dove (Streptopelia turtur)
- H. (Parahaemoproteus) vacuolatus — yellow whiskered greenbul (Andropadus latirostris)
- H. (Parahaemoproteus) valkiūnasi — great frigatebirds (Fregata minor), lesser frigatebirds (Fregata ariel), Ascension frigatebirds (Fregata aquila)[13]
- H. vangii — hook billed vanga (Vanga curvirostris)
- H. (Parahaemoproteus) velans — red-bellied woodpecker (Melanerpes carolinus), red-cockaded woodpecker (Picoides borealis)
- H. zosteropsis — oriental white eye (Zosterops palpebrosa palpebrosa)
Reptile hosts
- H. anatolicum — tortoise (Testudo graeca)
- H. balli — Egyptian cobra (Naja haje haje)
- H. chelodina — saw-shelled tortoise (Elseya latisternum)
- H. edomensis — lizard (Agama stellio)
- H. geochelonis — tortoise (Geochelone denticulata)
- H. kopki — spotted Indian house gecko (Hemidactylus brookei), giant frog eye gecko (Teratoscincus scincus)
- H. mackerrasi — Binoe's prickly gecko (Heteronotia binoei)
- H. mesnili — spitting cobra (Naja nigricollis nigricolli)
- H. metchnikovi — turtle (Chrysemys picta), yellow bellied terrapin (Tramchemys scripta)
- H. oedurae — Australian northern velvet gecko (Oedura castelnaui)
- H. peltocephali — river turtle (Peltocephalus dumerilianus)
- H. phyllodactyli — gekkonid (Ptyodactylus elisa)
- H. ptyodactyli — Kramer's yellow fan-fingered gecko (Ptyodactylus hasselquistii)
- H. tarentolae — Moorish gecko (Tarentola mauritanica)
- H. trionyxi — Ganges softshell turtle (Trionyx gangeticus)
Amphibian hosts
- H. ovalis — cricket frog (Rana limnocharis)
Hosts known to be infected but Haemoproteus species not identified
- common myna (Acridotheres tristis)
- Blyth's reed warbler (Acrocephalus dumetorum)
- sedge warblers (Acrocephalus schoenobaenus)
- reed warbler (Acrocephalus scirpaceus)
- clamorous reed warbler (Acrocephalus stentoreus)
- black throated sunbird (Aethopyga saturata)
- Spanish red-legged partridge (Alectoris rufa)
- imperial eagles (Aquila heliaca)
- canvasbacks (Aythya valisineria)
- white cockatoo (Cacatua alba)[14]
- sulphur-crested cockatoo (Cacatua galerita)[14]
- speckled pigeon (Columba guinea)
- white-rumped shama (Copsychus malabaricus)
- green jays (Cyanocorax yncas glaucescens)
- European bee-eaters (Merops apiaster)
- mute swan (Cygnus olor)
- magnificent bird of paradise (Diphyllodes magnificus hunsteini)
- red munia (Estrilda amandava)
- lesser kestrel (Falco naumanni)
- common kestrel (Falco tinnunculus)
- Swainson's francolin (Francolinus swainsonii)
- magnificent frigatebirds (Fregata magnificens)
- chaffinch (Fringilla coelebs)
- hill mynah (Gracula religiosa intermedia)
- long tailed shrike (Lanius schach)
- superb bird of paradise (Lophorina superba)
- Egyptian kites (Milvus migrans aegypticus)
- Guianan red-capped cardinal (Paroaria gularis gularis)
- lesser flamingos (Phoeniconaias minor)
- New Holland honeyeaters (Phylidonyris novaehollandiae)
- streaked weaver (Ploceus manyar)
- Surinam crested oropendola (Psarocolius decumanus decumanus)
- Montezuma oropendolas (Psarocolius montezuma)
- Guianan turquoise tanager (Tangara mexicana mexicana)
- blue-necked tanager (Tangara cyanicollis caeruleocephala)
- sacred ibis (Threskiornis aethiopicus)
- white-crowned sparrows (Zonotrichia leucophrys oriantha)
Vectors
- H. balmorali — Culicoides impunctatus
- H. belopolskyi — Culicoides impunctatus
- H. columbae — Ornithomyia avicularia, Pseudolynchia canariensis
- H. danilewskyi — Culicoides arboricola, Culicoides edeni, Culicoides knowltoni
- H. dolniki — Culicoides impunctatus
- H. fringillae — Culicoides impunctatus
- H. lanii — Culicoides impunctatus
- H. lophortyx — Culicoides bottimeri, Lynchia hirsuta, Stilbometopa impressa
- H. metchinikovi — Chrysops callidus
- H. nettionis — Culicoides downesi
- H. sacharovi — Peseudolynchia maura
- H. syrnii — Ornithomyia avicularia
- H. tartakovskyi — Culicoides impunctatus
- H. turtur — Pseudolynchia canariensis
Avian families affected
The concept of a "one host-one species" was originally used in the taxonomy of this genus as it appears that the parasites are at least moderately host specific. After this rule was found to be incorrect, it was suggested that the avian parasite species were limited to single avian families. From an inspection of the host records above it is clear that this is not the case.
The avian species known to be infected are listed below:
Order Accipitriformes
Family Accipitridae
- Cooper's hawk (Accipiter cooperii)
- Sharp shinned hawk (Accipiter striatus)
- Eastern imperial eagle (Aquila heliaca)
- White-backed vulture (Gyps africanus)
- Black kite (Milvus migrans)
- Hooded vulture (Necrosyrtes monachus)
- White-headed vulture (Trigonoceps occipitalis)
- Lappet faced vulture (Torgos tracheliotos)
Family Cathartidae
- Turkey vulture (Cathartes aura)
Order Anseriformes
Family Anatidae
- Wood duck (Aix sponsa)
- Blue winged teal (Anas discors)
- Mallard duck (Anas platyrhynchos)
- Lesser scaup (Aythya affinis)
- Ring necked duck (Aythya collaris)
- Canvasback (Aythya valisineria)
- Muscovy duck (Cairina moschata)
- Trumpeter swan (Cygnus buccinator)
- Mute swan (Cygnus olor)
- Black-bellied whistling duck (Dendrocygna autumnalis)
- Common merganser (Mergus merganser)
Order Charadriiformes
Family Laridae
- Swallow tailed gull (Creagrus furcatus)
- Caspian gull (Larus cachinnans)
Order Ciconiiformes
Family Ciconiidae
- Wood stork (Mycteria americana)
Order Columbiformes
Family Columbidae
- Speckled pigeon (Columba guinea)
- Japanese wood pigeon (Columba janthina)
- Rock pigeon (Columba livia)
- Common wood pigeon (Columba palumbus)
- Socorro common ground dove (Columbina passerina socorroensis)
- Ruddy ground dove (Columbina talpacoti)
- Tambourine dove (Turtur timpanistria)
- Scaled dove (Scardafella squammata)
- European turtle dove (Streptopelia turtur)
- Laughing dove (Stigmatopelia senegalensis)
- White-winged dove (Zenaida asiatica)
- Eared dove (Zenaida auriculata)
- Galápagos Dove (Zenaida galapagoensis)
- Mourning dove (Zenaida macroura)
Order Coraciiformes
Family Alcedinidae
- African pygmy kingfisher (Ispidina picta)
Family Brachypteraciidae
- Rufous headed ground roller (Atelornis crossleyi)
- Pitta like ground roller (Atelornis pittoides)
Family Bucerotidae
- Red-billed hornbill (Tockus erythrorhynchus)
Family Meropidae
- Blue checked bee-eater (Merops superciliosus)
Order Falconiformes
Family Falconidae
- Saker falcon (Falco cherrug)
- Lesser kestrel (Falco naumanni)
- American kestrel (Falco sparverius)
- Common kestrel (Falco tinnunculus)
- Chimango caracara (Milvago chimango)
Order Galliformes
Family Numididae
- Helmeted guineafowl (Numida meleagris)
Family Odontophoridae
- California quail (Callipepla californica)
- Scaled quail (Callipepla squamata)
- Bobwhite quail (Colinus virginianus)
Family Phasianidae
- Red legged partridge (Alectoris rufa)
- Chinese bamboo partridge (Bambusicola thoracicus)
- Ahanta francolin (Francolinus ahantensis)
- Swainson's francolin (Francolinus swainsonii)
- Chicken (Gallus gallus)
- Willow grouse (Lagopus lagopus)
- Wild turkey (Meleagris gallopavo)
Family Tetraonidae
- Dusky grouse (Dendragapus obscurus
Order Gruiformes
Family Gruidae
- Black crowned crane (Balearica pavonina)
- Sandhill crane (Grus canadensis)
Family Otidae
- MacQueen's bustard (Chlamydotis macqueenii)
- Red crested bustard (Eupodotis ruficrista)
- Great bustard (Otis tarda)
Order Passeriformes
Family Acrocephalidae
- Great reed warbler (Acrocephalus arundinaceus)
- Blyth's reed warbler (Acrocephalus dumetorum)
- Marsh warbler (Acrocephalus palustris)
- Sedge warbler (Acrocephalus schoenobaenus)
- Reed warbler (Acrocephalus scirpaceus)
- Clamorous reed warbler (Acrocephalus stentoreus)
Family Corvidae
- Blue jay (Cyanocitta cristata)
- Green jay (Cyanocorax yncas)
Family Dicruridae
- Fork-tailed drongo (Dicrurus adsimilis)
- Crested drongo (Dicrurus forficatus)
Family Emberizidae
- Dark eyed junco (Junco hyemalis)
- Rufous winged sparrow (Peucaea carpalis)
- White crowned sparrow (Zonotrichia leucophrys)
Family Estrildidae
- Red munia (Amandava amandava)
- Indian silverbill (Euodice malabarica)
- Tricoloured munia (Lonchura malacca)
- Scaly breasted munia (Lonchura punctulata)
Family Fringillidae
- House finch (Carpodacus mexicanus)
- Hawfinch (Coccothraustes coccothraustes)
- Chaffinch (Fringilla coelebs)
Family Hirundinidae
- Purple martin (Progne subis)
Family Icteridae
- Crested oropendola (Psarocolius decumanus)
- Montezuma oropendola (Psarocolius montezuma)
Family Laniidae
- Red backed shrike (Lanius collurio)
- Long tailed shrike (Lanius schach)
- Woodchat shrike (Lanius senator)
Family Meliphagidae
- New Holland honeyeaters (Phylidonyris novaehollandiae)
Family Mimidae
- Gray catbird (Dumetella carolinensis)
Family Motacillidae
- Yellow wagtail (Motacilla flava)
Family Muscicapidae
- White rumped shama (Copsychus malabaricus)
- Oriental magpie robin (Copsychus saularis)
Family Nectariniidae
- Black throated sunbird (Aethopyga saturata)
- Olive sunbird (Cyanomitra olivacea)
Family Oriolidae
- Golden oriole (Oriolus oriolus)
Family Paridae
- Blue tit (Cyanistes caeruleus)
Family Paradisaeidae
- Magnificent bird of paradise (Cicinnurus magnificus)
- Superb bird of paradise (Lophorina superba)
Family Parulidae
- American redstart (Setophaga ruticilla)
Family Passeridae
- House sparrow (Passer domesticus)
Family Ploceidae
- Red headed malimbe (Malimbus rubricollis)
- Streaked weaver (Ploceus manyar)
- Black-headed weaver (Ploceus melanocephalus)
- Baya weaver (Ploceus philippinus)
- Red billed quelea (Quelea quelea)
Family Pycnonotidae
- Yellow whiskered bulbul (Andropadus latirostris)
- Red whiskered bulbul (Pycnonotus jocosus)
Family Sturnidae
- Common myna (Acridotheres tristis)
- Common hill myna (Gracula religiosa)
- Chestnut tailed starling (Sturnia malabarica)
- Asian pied starling (Sturnus contra)
- Greater blue eared glossy starling (Lamprotornis chalybaeus)
Family Sylviidae
- Blackcap (Sylvia atricapilla)
Family Thraupidae
- Bananaquit (Coereba flaveola)
- Red capped cardinal (Paroaria gularis)
- Blue necked tanager (Tangara cyanicollis)
- Turquoise tanager (Tangara mexicana)
Family Timaliidae
- Rufous sibia (Heterophasia capistrata)
- Jungle babbler (Turdoides striata)
Family Turdidae
- Swainson's thrush (Catharus ustulatus)
Family Vangidae
- Hook billed vanga (Vanga curvirostris)
Family Zosteropidae
- Oriental white eye (Zosterops palpebrosus)
Order Pelecaniformes
Family Fregatidae
- Magnificent frigatebird (Fregata magnificens)
- Great frigatebird (Fregata minor)
Family Threskiornithidae
- African sacred ibis (Threskiornis aethiopicus)
- Glossy ibis (Plegadis falcinellus)
Order Piciformes
Family Megalaimidae
- Coppersmith barbet (Megalaima haemacephala)
Family Picidae
- Red bellied woodpecker (Melanerpes carolinus)
- Red cockaded woodpecker (Picoides borealis)
- Grey faced woodpecker (Picus canus)
- Greater yellownape (Picus flavinucha)
Order Phoenicopteriformes
Family Phoenicopteridae
- Lesser flamingo (Phoenicopterus minor)
Order Psittaciformes
Family Cacatuidae
- White cockatoo (Cacatua alba)
- Sulphur crested cockatoo (Cacatua galerita)
- Yellow crested cockatoo (Cacatua sulphurea)
Family Psittacidae
- Plum headed parakeet (Psittacula cyanocephala)
- Rose ringed parakeet (Psittacula krameri)
- Blossom headed parakeet (Psittacula roseata)
- African grey parrot (Psittacus erithacus)
Order Strigiformes
Family Strigidae
- Snowy owl (Bubo scandiacus)
- Brown Hawk-Owl (Ninox scutulata)
- European scops owl (Otus scops)
- Brown owl (Strix aluco)
- Spotted owl (Strix occidentalis)
- Spotted wood owl (Strix seloputo)
Notes
Haemoproteus balazuci Dias 1953 is a junior synonym of Haemoproteus testudinalis
Haemoproteus gymnorhidis de Mello 1936, Haemoproteus granulosum Rey Vila 1945, Haemoproteus danilewskyi var. urbanensis Sachs 1953 and Haemoproteus zasukhini Burtikashvili 1973 are considered to be synonyms of Haemoproteus passeris Kruse 1890.
Haemoproteus rouxi Novy and MacNeal 1904 is a nomen nudum.
References
- Poinar G, Telford SR (2005). "Paleohaemoproteus burmacis gen. n., sp. n. (Haemospororida: Plasmodiidae) from an Early Cretaceous biting midge (Diptera: Ceratopogonidae)". Parasitology. 131 (1): 79–84. doi:10.1017/S0031182005007298. PMID 16038399.
- Telford, SR (1996). "Two new species of Haemocystidium Castellani & Willey (Apicomplexa: Plasmodiidae) from Pakistani lizards, and the support their meronts provide for the validity of the genus". Systematic Parasitology. 34 (3): 197–214. doi:10.1007/bf00009387.
- Clark, Nicholas; Clegg, Sonya; Lima, Marcos (2014). "A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data". International Journal for Parasitology. 44 (5): 329–338. doi:10.1016/j.ijpara.2014.01.004. hdl:10072/61114. PMID 24556563.
- Levin, II; Valkiūnas, G; Santiago-Alarcon, D; Cruz, LL; Iezhova, TA; O'Brien, SL; Hailer, F; Dearborn, D; Schreiber, EA; Fleischer, RC; Ricklefs, RE; Parker, PG (2015). "Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: evidence from molecular and morphological studies, with a description of Haemoproteus iwa". Int J Parasitol. 41 (10): 1019–27. doi:10.1016/j.ijpara.2011.03.014. PMID 21683082.
- Markus, MB; Oosthuizen, JH (1972). "Pathogenicity of Haemoproteus columbae". Transactions of the Royal Society of Tropical Medicine and Hygiene. 66 (1): 186–187. doi:10.1016/0035-9203(72)90072-7. PMID 4625895.
- Clark, Nicholas; Adlard, Robert; Clegg, Sonya (2014). "First evidence of avian malaria in Capricorn Silvereyes (Zosterops lateralis chlorocephalus) on Heron Island". The Sunbird. 44: 1–11.
- Waite, JL; Henry, AR; Adler, FR; Clayton, DH (2012). "Sex-specific effects of an avian malaria parasite on an insect vector: support for the resource limitation hypothesis". Ecology. 93 (11): 2448–55. doi:10.1890/11-2229.1. PMID 23236915.
- Iezhova TA, Valkiūnas G, Loiseau C, Smith TB, Sehgal RN (2010). "Haemoproteus cyanomitrae sp. nov. (Haemosporida: Haemoproteidae) from a widespread African songbird, the olive sunbird, Cyanomitra olivacea". J. Parasitol. 96 (1): 137–143. doi:10.1645/GE-2198.1. PMID 19691417.
- Elahi, Rubayet; Islam, Ausraful; Hossain, Mohammad Sharif; Mohiuddin, Khaja; Mikolon, Andrea; Paul, Suman Kumer; Hosseini, Parviez Rana; Daszak, Peter & Alam, Mohammad Shafiul (2014). "Prevalence and Diversity of Avian Haematozoan Parasites in Wetlands of Bangladesh". Journal of Parasitology Research. 2014. doi:10.1155/2014/493754.
- Karadjian, G.; Martinsen, E.; Duval, L.; Chavatte, J.-M.; Landau, I. (2014). "Haemoproteus ilanpapernai n. sp. (Apicomplexa, Haemoproteidae) in Strix seloputo from Singapore: morphological description and reassignment of molecular data". Parasite. 21: 17. doi:10.1051/parasite/2014018. PMC 3996868. PMID 24759652.
- Križanauskiene A, Pérez-Tris J, Palinauskas V, Hellgren O, Bensch S, Valkiūnas G (2010). "Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov". Parasitology. 137 (2): 217–27. doi:10.1017/S0031182009991235. PMID 19765350.
- Karadjian, G.; Puech, M.-P.; Duval, L.; Chavatte, J.-M.; Snounou, G.; Landau, I. (2013). "Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species". Parasite. 20: 32. doi:10.1051/parasite/2013031. PMC 3771403. PMID 24029169.
- Merino, Santiago; Hennicke, Janos; Martínez, Javier; Ludynia, Katrin; Torres, Roxana; Work, Thierry M.; Stroud, Stedson; Masello, Juan F.; Quillfeldt, Petra (2012). "Infection by Haemoproteus parasites in four species of frigatebirds and the description of a new species of Haemoproteus (Haemosporida: Haemoproteidae)" (PDF). J. Parasitol. 98 (2): 388–97. doi:10.1645/GE-2415.1. PMID 21992108.
- Cordon, G.; Hitos Prados, A.; Romero, D.; Sánchez Moreno, M.; Pontes, A.; Osuna, A.; Rosales, M.J.; et al. (2009). "Intestinal and haematic parasitism in the birds of the Almunecar (Granada, Spain) ornithological garden". Veterinary Parasitology. 165 (3–4): 361–6. doi:10.1016/j.vetpar.2009.07.027. PMID 19682800.