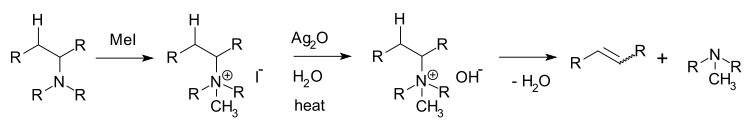
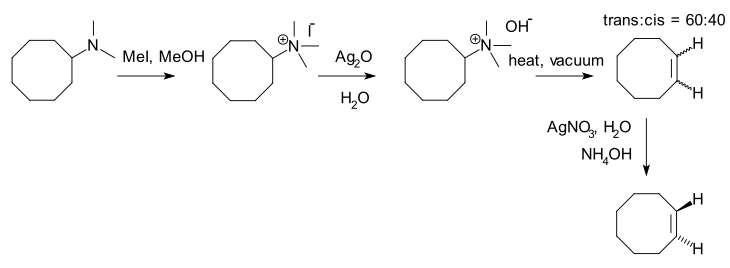
Hofmann elimination
Hofmann elimination is an elimination reaction of an amine where the least stable (least substituted) alkene, the Hofmann product, is formed. This tendency, known as the Hofmann alkene synthesis rule, is in contrast to usual elimination reactions, where Zaitsev's rule predicts the formation of the most stable alkene. It is named after its discoverer, August Wilhelm von Hofmann.[1][2]
| Hofmann elimination | |
|---|---|
| Named after | August Wilhelm von Hofmann |
| Reaction type | Elimination reaction |
| Identifiers | |
| Organic Chemistry Portal | hofmann-elimination |
| RSC ontology ID | RXNO:0000166 |
The reaction involves the formation of a quaternary ammonium iodide salt by treatment of the amine with excess methyl iodide (exhaustive methylation), followed by treatment with silver oxide and water to form a quaternary ammonium hydroxide. When this salt is decomposed by heat, the Hofmann product is preferentially formed due to the steric bulk of the leaving group causing the hydroxide to abstract the more easily accessible hydrogen.
An example is the synthesis of trans-cyclooctene:[3]
In a related chemical test, known as the Herzig–Meyer alkimide group determination, a tertiary amine with at least one methyl group and lacking a beta-proton is allowed to react with hydrogen iodide to the quaternary ammonium salt which when heated degrades to iodomethane and the secondary amine.[4]
See also
References
- Hofmann, A. W. (1851). "Researches into the molecular constitution of the organic bases". Philosophical Transactions of the Royal Society of London. 141: 357–398. doi:10.1098/rstl.1851.0017. S2CID 108453887.
- Aug. Wilh. von Hofmann (1851). "Beiträge zur Kenntniss der flüchtigen organischen Basen" [Contribution to [our] knowledge of volatile organic bases]. Annalen der Chemie und Pharmacie (in German). 78 (3): 253–286. doi:10.1002/jlac.18510780302.
- Arthur C. Cope; Robert D. Bach (1973). "trans-Cyclooctene". Organic Syntheses.; Collective Volume, 5, p. 315
- J. Herzig; H. Meyer (1894). "Ueber den Nachweis und die Bestimmung des am Stickstoff gebundenen Alkyls". Berichte der deutschen chemischen Gesellschaft. 27 (1): 319–320. doi:10.1002/cber.18940270163.