Humulone
Humulone (α-lupulic acid), a vinylogous type of organic acid, is a bitter-tasting chemical compound found in the resin of mature hops (Humulus lupulus).[2] Humulone is a prevalent member of the class of compounds known as alpha acids, which collectively give hopped beer its characteristic bitter flavor.
-Humulone.svg.png.webp) | |
 | |
| Names | |
|---|---|
| IUPAC name
(6S)-3,5,6-Trihydroxy-2-(3-methylbutanoyl)-4,6-bis(3-methylbut-2-en-1-yl)cyclohexa-2,4-dien-1-one[1] | |
| Other names
α-Lupulic acid; α-Bitter acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.043.371 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O5 | |
| Molar mass | 362.466 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
In terms of structure, humulone is a phloroglucinol derivative with three isoprenoid side-chains. Two side-chains are prenyl groups and one is an isovaleryl group. The acidity of the ring enol moieties that give rise to its designation as an acid lie in their vinylogous relationship with the ring and side chain carbonyl functional groups.
Isohumulone
During the brewing process, humulone degrades to cis- and trans-isohumulone.[1] These “alpha acids” survive the boiling process, although numerous oxidized derivatives are produced.[3] The iso-alpha acids are significantly more soluble than humulone at the pH levels typically present in the brewing process.[4]
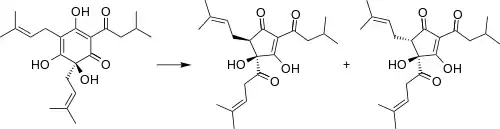 Degradation of humulone to cis- and trans-isohumulone
Degradation of humulone to cis- and trans-isohumulone
Laboratory synthesis
Humulone can be synthesized by the acylation of benzene-1,2,3,5-tetrol with isovaleryl chloride to give 2,3,4,6-tetrahydroxyisovalerophenone. This step is followed by prenylation with 1-bromo-3-methyl-2-butene to give humulone.[5]
 Synthesis of humulone from benzene-1,2,3,5-tetrol
Synthesis of humulone from benzene-1,2,3,5-tetrol
Biosynthesis
As determined by INADEQUATE 2D NMR, the biosynthesis of humulone in Humulus lupulus starts with an isovaleryl-CoA unit and 3 malonyl-CoA units catalyzed by phlorovalerophenone synthase. This conversion yields the benzenoid 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one. Dimethylallyl pyrophosphate is then obtained from the deoxyxylulose pathway, where prenylation of the benzenoid occurs, yielding humulone.[6]
- isovaleryl-CoA + 3 malonyl-CoA → 4 CoASH + 3 CO2 + 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one
- 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one + 2 DMAPP →C21H30O5
Research
Humulone is under basic research with in vitro studies to determine if it has biological properties, such as possible GABAA receptor activity[7][8] or antibacterial effects.[9]
References
- Urban, Jan; Dahlberg, Clinton; Carroll, Brian; Kaminsky, Werner (2013). "Absolute Configuration of Beer′s Bitter Compounds". Angew. Chem. Int. Ed. 52 (5): 1553–1555. doi:10.1002/anie.201208450. PMC 3563212. PMID 23239507.
- De Keukeleire, J; Ooms, G; Heyerick, A; Roldan-Ruiz, I; Van Bockstaele, E; De Keukeleire, D (2003). "Formation and accumulation of alpha-acids, beta-acids, desmethylxanthohumol, and xanthohumol during flowering of hops (Humulus lupulus L.)". Journal of Agricultural and Food Chemistry. 51 (15): 4436–41. doi:10.1021/jf034263z. PMID 12848522.
- Blanco, C. A.; Rojas, A; Caballero, P. A.; Ronda, F; Gomez, M; Caballero, I., “A better control of beer properties by predicting acidity of hop iso-α-acids”, Trends in Food Science & Technology, Volume 17, Issue 7, July 2006, Pages 373-377, ISSN 0924-2244, doi:10.1016/j.tifs.2005.11.012.
- Esslinger, H. M. and Narziss, L. 2003. “Beer.” in- Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, 2009 doi:10.1002/14356007.a03_421
- Obara; et al. (1989). "A Synthetic Route to (±)-humulone". Bull. Chem. Soc. Jpn. 62 (9): 3034–3035. doi:10.1246/bcsj.62.3034.
- M. Goese; K. Kammhuber; A. Bacher; M. H. Zenk; W. Eisenreich (1999). "Biosynthesis of bitter acids in hops. A (13)C-NMR and (2)H-NMR study on the building blocks of humulone". FEBS Journal. 263 (2): 447–454. doi:10.1046/j.1432-1327.1999.00518.x. PMID 10406953.
- Benkherouf, Ali Y.; Eerola, Kim; Soini, Sanna L.; Uusi-Oukari, Mikko (2020). "Humulone Modulation of GABAA Receptors and Its Role in Hops Sleep-Promoting Activity". Frontiers in Neuroscience. 14. doi:10.3389/fnins.2020.594708. ISSN 1662-453X.
- Benkherouf, Ali Y.; Logrén, Nora; Somborac, Tamara; Kortesniemi, Maaria; Soini, Sanna L.; Yang, Baoru; Salo-Ahen, Outi M.H.; Laaksonen, Oskar; Uusi-Oukari, Mikko (2020-04-01). "Hops compounds modulatory effects and 6-prenylnaringenin dual mode of action on GABAA receptors". European Journal of Pharmacology. 873: 172962. doi:10.1016/j.ejphar.2020.172962. PMID 32001220.
- Yamaguchi, N; Satoh-Yamaguchi, K; Ono, M (2009). "In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris". Phytomedicine. 16 (4): 369–76. doi:10.1016/j.phymed.2008.12.021. PMID 19201179.
